Abstract
Sea ice, frozen seawater, is more than a “white desert” in the Earth’s polar regions. The solid part of sea ice is mostly pure ice, similar to what you could make by putting tap water in a freezer. It also contains an intricate network of pores, pockets, and channels—known as a brine network—which develops each season. The brine network is filled with a very salty solution that contains nutrients that ice-associated organisms (bacteria, algae, and small animals) use as food. Algae are especially important because they remove carbon dioxide from the atmosphere and provide some of the oxygen we breathe every day. However, ice organisms are witnessing the consequences of human pollution and climate change. Although the polar regions are located far from human areas, the ocean circulation carries pollutants to the poles. This article examines what is happening in the seemingly inhospitable but crowded brine network, including the latest observations on the accumulation of human pollution.
Sea-Ice: A Constantly Changing Environment
During Antarctic wintertime, a large part of the Southern Ocean is covered with a white “blanket”, called sea ice. Sea ice is frozen seawater, containing so little salt that you would hardly notice if you tasted it. When water freezes, most of the salt from seawater does not become part of the ice itself, but ends up inside channels and pockets within the ice, called a brine network. Brine is the name for water with a lot of salt dissolved in it, and the saltier the brine, the lower the temperature required to freeze it. Saltiness is the reason why seawater freezes at −1.8°C, while pure water freezes at 0°C.
Temperature varies within sea ice, and so does the structure of the brine network. Small brine pockets are stable in winter, when ice can get as cold as −30°C due to very low air temperatures. In spring, sea ice warms up, reaching approximately 0°C, and pockets open into channels [1]. The resulting structure has tree-like branches (Figure 1a). While sea ice is growing during autumn and winter, these channels exist in the 10–20 cm of ice closest to the water [2]. It is relatively warm in this bottom layer because the seawater below the ice can usually only get as cold as −1.8°C [3]. When the upper parts of the sea ice warm up in spring, channels can extend from the top of the ice all the way to the bottom. The channels grow from the top of the ice to the bottom due to saltiness: salt is heavier than water, which means that brine is heavier than both ice and seawater. As the ice warms, brine melts it and moves downwards due to gravity. Some seawater can move upward to replace the downward moving brine, bringing nutrients, dissolved gases, and small organisms, into the ice. Sea ice is thus a very active, constantly changing environment (Figure 1b).
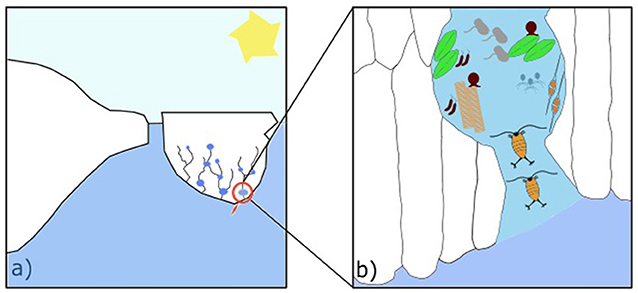
- Figure 1 - (a) A piece of sea ice, called an ice floe, with brine channels (the string-like structure) and pockets (any enlargement along the channels) near the bottom.
- (b) A zoomed image shows two copepods moving across a brine channel, reaching other organisms in a brine pocket. Sizes do not represent the real dimensions of the organisms—they are actually extremely tiny.
Who Lives Inside Sea Ice?
Each season, the cycle of freezing and melting and the formation of the brine network means that only tiny organisms that are very flexible to changes in temperature, salinity, and light can grow in sea-ice channels. Since the temperature in sea ice is very low, organisms produce anti-freeze compounds to prevent their cells from freezing—the same compounds we use to soften ice cream! Other organisms produce substances that help them cope with changing salinity. But why would any organism want to live in sea ice? Well, the brine pockets are very small and larger organisms cannot get in, so they can provide shelter for tiny organisms. The community within brine pockets consists of invertebrates, such as very tiny copepods, or worm-like organisms known as nematodes. We also find microorganisms such as microalgae, bacteria, or viruses (Figure 1b). While copepods are big enough to be spotted with the naked eye, other organisms are so small that you need a microscope to see their fascinating shapes. When a chunk of ice is tipped over, its underside appears brown (Figure 2a). The brown color comes from tiny ice-associated algae called diatoms. To slide through the brine channels, diatoms eject slimy stuff through an opening in their shells. Some diatoms build long chains (Figure 2b) or produce mucus around small single cells (Figure 2c) or have shells with interesting shapes (Figure 2d).
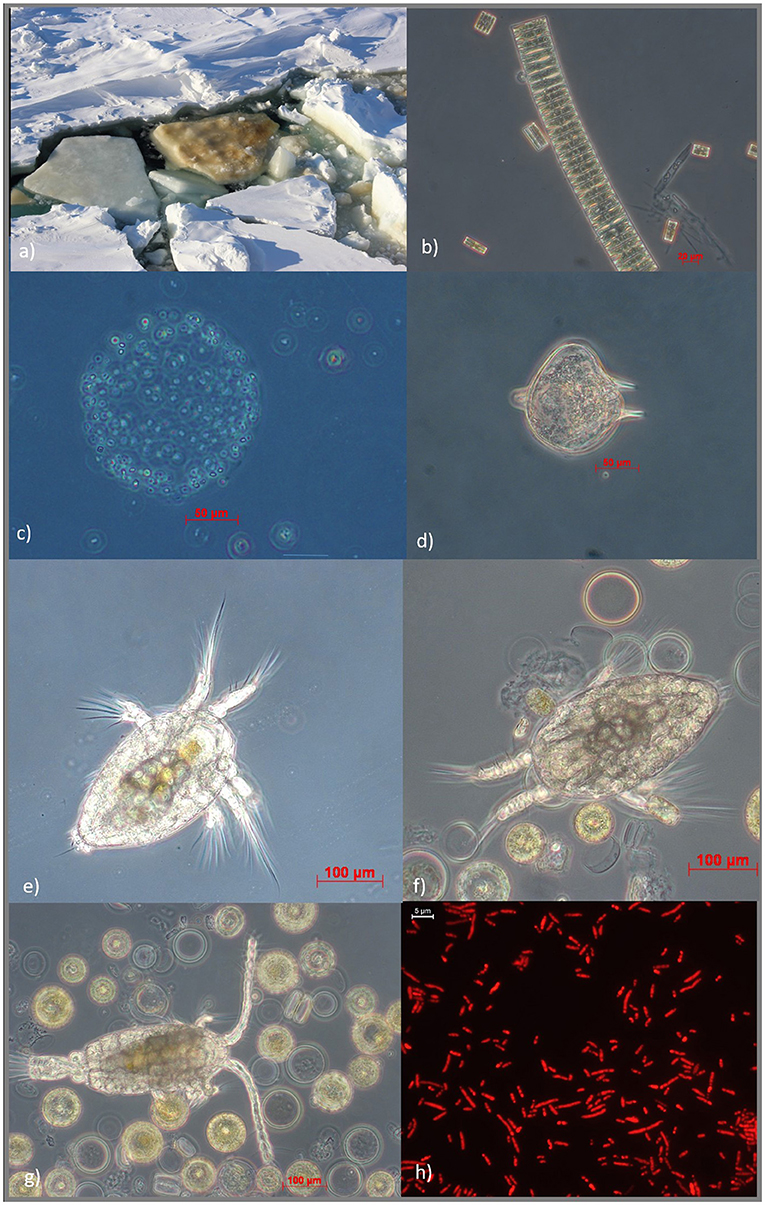
- Figure 2 - (a) A flipped-over piece of sea ice reveals a brown color underneath, due to growth of sea-ice microalgae, likely diatoms; (b) a typical chain-forming type of diatom; (c) foam algae, in which very tiny cells live in a ball-shaped colony; (d) a small dinoflagellate with little “horns”; (e, f) “baby” copepods; (g) a copepod eating diatoms; and (h) sea-ice bacteria stained with a red dye to make them more visible (image credits: (a) Mareike Bach; (b–g) Ilka Peeken (@AWI); (h) Eeva Eronen-Rasimus).
The Important Role of Sea-Ice Algae
Algae are important because they remove carbon dioxide from the atmosphere and provide about half of the oxygen we breathe every day. Algae are the base of the polar food web. Through photosynthesis, they “eat” sunlight, carbon dioxide, and water to produce food for themselves—and eventually for other organisms that eat them. Algae are eaten by small animals called zooplankton, like copepods (Figures 2e–g), and zooplankton are then eaten by fish. When there are not enough nutrients in the brine channels, algae eventually die; bacteria (Figure 2h) and fungi start eating the dead material, such as the slimy stuff released by diatoms. This process is called decomposition, and it provides fresh nutrients that can then be used again by organisms living nearby. This efficient recycling system within the ice is called a microbial loop, and it is a very important way to bring carbon back to other organisms in the food web. Overall, sea-ice organisms have an important role in polar marine ecosystems as a rich food source, forming the base of the polar food web.
Microplastics: A Major Threat for Microorganisms and the Environment
The sea-ice ecosystem is under threat from man-made pollution. The polar regions are far away from cities and factories, but we find pollution from other parts of the world within the ice. One of these is plastic, recently identified as a major threat to aquatic (water-based) ecosystems worldwide. About 40% of plastic in the oceans is single-use plastic (bottles, cutlery, bags, etc.). The remaining 60% is mostly waste from our homes (washing), factories, or fishing gear. Cities and coastal areas have the highest amounts of plastics; however, rivers, groundwater, ocean currents, and currents in the atmosphere can carry plastics all the way to remote polar areas (Figure 3). During this travel, physical and chemical processes (sunlight, high temperatures, wind and wave action, and microbial decomposition) break down plastics, resulting in very small plastic fragments. When the fragments are smaller than 0.5 cm, they are called microplastics. When sea ice forms, microplastics can end up accumulating within it [4].
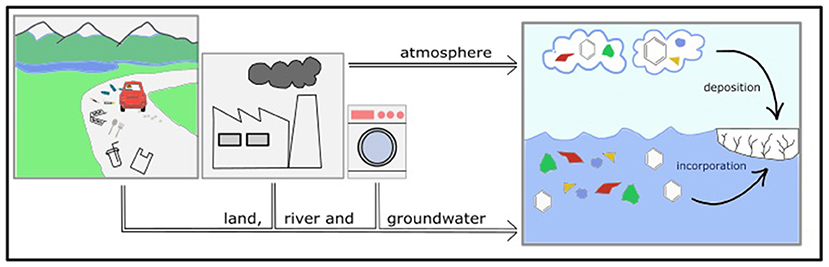
- Figure 3 - Plastics and persistent organic pollutants can make their way from their production sites to the oceans.
- Lots of single-use plastic can be found wherever humans are found. Breakdown produces microplastics (colored polygons), which meet up with more plastics and persistent organic pollutants (hexagons) that come from industrial processes, as well as washing and farming. Microplastics and persistent organic pollutants found in land, rivers, and groundwater can reach the polar ocean and enter the ice. Microplastics and persistent organic pollutants can also be transported through the atmosphere and the oceans, reaching the polar regions and entering the ice.
The microplastic particles in sea ice are the same size as diatoms and therefore can be eaten by very small animals, such as copepods. The consequences can be serious: due to their small size, microplastics can clog an organism’s internal pathways or act as food, filling the stomach and causing the organism to starve to death. Microplastics can harm algae so they cannot perform photosynthesis, microplastic particles can compete for space in brine channels. Microplastics also offer “free rides” to species that do not belong in the polar ocean and can compete with local species. Furthermore, plastics can release some chemicals that are harmful to animals. A recent United Nations report highlights that there are 51 trillion microplastic particles in the seas, and these numbers are increasing [5]. Researchers are still trying to understand the paths that microplastics take and the effects they can have. From what we know now, it is important to use less plastic so that less of it ends up in the environment.
Other Dangerous Pollutants
Another type of life-threatening compound present in sea ice is called persistent organic pollutants. These compounds were first produced in the 1920s and 1930s for use in industries (e.g., burning of waste, steel industry, road transport) and agriculture (e.g., insecticides, pesticides). Air and ocean circulation carry persistent organic pollutants to the polar regions. These compounds can turn into vapor (gas) at high temperatures (near the equator) and then turn back to liquid at low temperatures (in polar regions). They are called “persistent” because they can remain in soil and water for long periods, and they are very difficult to get rid of [6].
Persistent organic pollutants are extremely dangerous to polar organisms because these substances can build up in fat and polar animals are very fatty—fat allows them to survive the long, cold winter. Small polar organisms have a low persistent organic pollutant content, but the concentrations found in larger organisms (e.g., super-predators like sharks, orcas, and even humans), which feed on smaller contaminated organisms, can be up to 200,000 times the ocean concentration! This process of increasing concentration along the food chain is called biomagnification, and it is very dangerous. Eating fatty tissues containing persistent organic pollutants can cause problems with the immune system, reproductive system, and nervous system, as well as behavioral abnormalities. As climate change and melting of Antarctic glaciers seems to be increasing the concentrations of these pollutants [6], 91 countries and the European community signed the Stockholm Convention in 2001, agreeing to reduce or eliminate the production of 12 key persistent organic pollutants. Although the number of pollutants in the agreement increased to 24 by 2017, developing countries are still producing these compounds for the rest of the world, so the problem is not solved.
Conclusion
Although sea ice is a very extreme habitat, many organisms are specifically adapted to live in this environment. Their ability to make homes in the tree-like structure of the sea-ice brine network is endangered, due to the accumulation of man-made pollution such as microplastics and persistent organic pollutants, as well as climate warming. Within sea ice, microplastics and persistent organic pollutants accumulate with as-yet unknown consequences for sea-ice organisms, which are an important food source in the Southern Ocean ecosystem. International agreements are trying to reduce the production of plastics and change industrial processes that produce dangerous pollutants. But people still act very carelessly—these pollutants and plastics are still accumulating in rivers and oceans. More “green” actions are needed, no matter where we live, to raise awareness of these threats and protect the sea ice and the organisms that live there.
Glossary
Brine Network: ↑ Highway within the ice characterized by cold and salty water, and hosting nutrients, dissolved gases, and organisms.
Salinity: ↑ Quantity (mass) of salts in a given volume of water.
Copepods: ↑ Tiny shrimp-like animals, part of the group of animals called crustaceans.
Diatoms: ↑ One-celled organisms that are one of the most common types of phytoplankton (i.e., tiny, “light-eating” organisms floating in the surface ocean). Diatoms can be single cells, or form chains or small groups called aggregates.
Microbial Loop: ↑ Cycle of organic material that is produced and decomposed by different microbial organisms
Microplastics: ↑ Pieces of plastic material smaller than 5 mm
Persistent Organic Pollutants: ↑ Substances present in concentrations that may harm humans, plants, and animals. Persistent organic pollutants can stick to fatty substances for a long time.
Biomagnification: ↑ Increase in concentration of a pollutant along the trophic chain.
Acknowledgments
IP was supported by the PoF IV program “Changing Earth - Sustaining our Future” Topic 6.4 of the German Helmholtz Association. MB was supported by the Netherlands Organisation for Scientific Research (NWO) under the Polar Program (NPP) Project Number ALWPP.2016.036.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Jardon, F., Vivier, F., Vancoppenolle, M., Lourenço, A., Bouruet-Aubertot, P., and Cuypers, Y. 2013. Full-depth desalination of warm sea ice. J. Geophys. Res.: Oceans. 118:435–47. doi: 10.1029/2012JC007962
[2] ↑ Eide, L. I., and Martin, S. 1975. The formation of brine drainage features in young sea ice. J. Glaciol. 14:137–54. doi: 10.3189/S0022143000013460
[3] ↑ Lake, R., and Lewis, E. 1970. Salt rejection by sea ice during growth. J. Geophysi. Res. 75:583–97. doi: 10.1029/JC075i003p00583
[4] ↑ Kelly, A., Lannuzel, D., Rodemann, T., Meiners, K., and Auman, H. 2020. Microplastic contamination in east Antarctic sea ice. Marine Pollut. Bullet. 154:111130. doi: 10.1016/j.marpolbul.2020.111130
[5] ↑ UN Environment. 2017. Combating Marine Plastic Litter and Microplastics: An Assessment of the Effectiveness of Relevant International, Regional and Subregional Governance Strategies and Approaches. Nairobi: UN Environment.
[6] ↑ Vorkamp, K., Carlsson, P., Corsolini, S., de Wit, C., Dietz, R., Gribble, M. O., et al. 2022. Influences of climate change on long-term time series of persistent organic pollutants (POPs) in Arctic and Antarctic biota. Environ. Sci. Process Impacts. 24:1643–60. doi: 10.1039/D2EM00134A

