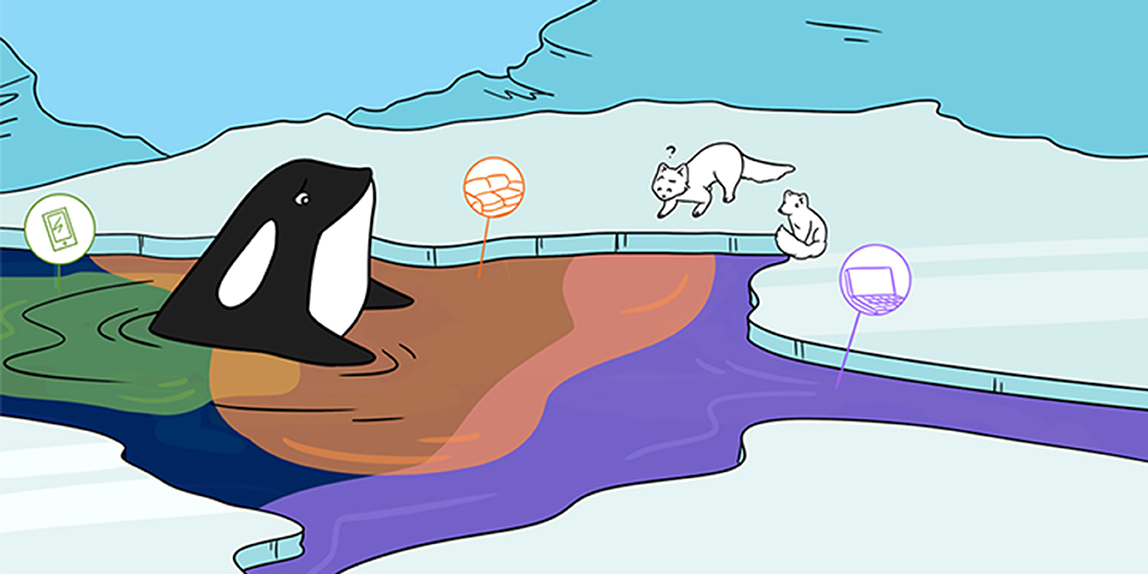
Abstract
It is hard to believe, but some of the chemicals in our couches, computers, and even phones can travel all the way to the Arctic. How is that possible? That is exactly what we were asking when we found chemicals that are used in everyday items—like computers, phones, and couches—in the Canadian Arctic. In this article, we will tell you about our research into these chemicals in the Canadian Arctic and what we found out about their abilities to “fly” and “swim” north to the Arctic. We will also share our ideas on how we can keep animals and people in the Arctic—and around the world—safe from some of these chemicals.
What Happens When Chemicals Do Not Stay Where They Are Supposed to Be?
The chemicals humans make can be fantastic! We can make chemicals that produce any smell, taste, or color we want. Chemicals are what makes medicine help people, chemicals make our cars drive and our planes fly.
Scientists called chemists make many new types of chemicals all the time. Many of these new chemicals are useful, but some can also be a problem. Chemicals are especially a problem if they do not stay where they are supposed to be but leach out from the products to which they are added.
Imagine, for example, a bright red shirt. The red color is great in the shirt, but after you wash it together with a white shirt, you might find that you have a red shirt and a pink shirt instead of a white one (do not try that out at home—your parents might get upset!). What happened? Some of the chemical that makes the red color did not stay where it was supposed to be, in the red shirt, but instead leached out into the water and colored the white shirt.
In the case of differently colored shirts, the leaching of chemicals is just annoying. But now imagine a medicine or a potentially dangerous chemical escaping from where it is supposed to be and entering the water. This chemical might then make animals living in or drinking that water sick.
We know that it can be very bad if large amounts of dangerous chemicals are spilled and reach the environment. But, unfortunately, even small amounts of chemicals can sometimes make animals and people sick.
To protect people and animals from chemicals that can harm them, governments around the world have agreed on ways to judge whether a chemical is too dangerous for use or if it can only be used in specific ways [1]. One of the important criteria to judge if a chemical is potentially dangerous is the time it takes for that chemical to degrade (break down), and finally disappear, in the environment.
Chemicals that degrade very slowly are called persistent. Some persistent chemicals can stay in the environment for hundreds of years. This means that if a persistent chemical can make a specific type of animal sick, it could continue doing so for a very long time, even for generations.
Why Are We Worried About Chemicals in the Arctic?
The Arctic is the high north of our earth. The Arctic is home to polar bears, seals, caribou, Arctic foxes, birds, fish, and people. But not many people live there because it is very cold and difficult to get to. There are also very few roads or shops and definitely no factories that produce chemicals.
Even though no chemicals are made in the Arctic, scientists have found many industrial chemicals in Arctic water, snow, air, and even the animals and people living there [2]. Some of these chemicals have been used by, for example, the oil and gas industry or military bases in the Arctic. But many of the chemicals the scientists found were not used in the Arctic.
So, the question was, “How do these chemicals get to the Arctic?” Scientists figured out that persistent chemicals can reach the Arctic from where they are made or used by traveling through the water and air.
Here is how these persistent chemicals reach the Arctic. They move more easily and quickly from places where it is warm, which are the places where most people live and where the factories are, but then the chemicals “get stuck” in areas where it is colder. So, persistent chemicals that can travel in water and air move north from where they were made until they get to the Arctic, and then they stay there [3].
This means that the animals and people in the Arctic—who hardly use these chemicals—are in contact with chemicals that could make them sick. Moreover, they do not even have any of the benefits from using the chemicals, or the choice of whether they want these chemicals around or not.
People Wanted to Make Things Better …
We are interested in certain chemicals called flame retardants. Flame retardants are used in many kinds of plastic to make sure they do not burn too easily. Flame retardants are used, for example, in computers, phones, carpets, and the foam in some couches and beds.
Unfortunately, many flame retardants that were used in the past, up until about 10 years ago, did not stay where they were supposed to be. Some of the flame retardants got out of the couches, phones, and all the other things they were used in, and got into the air and dust in houses, and then into the outside air and water [4] (Figure 1). The worst part was that these flame retardants were persistent, could reach the Arctic, and were harmful—so they could make people and animals sick [5].
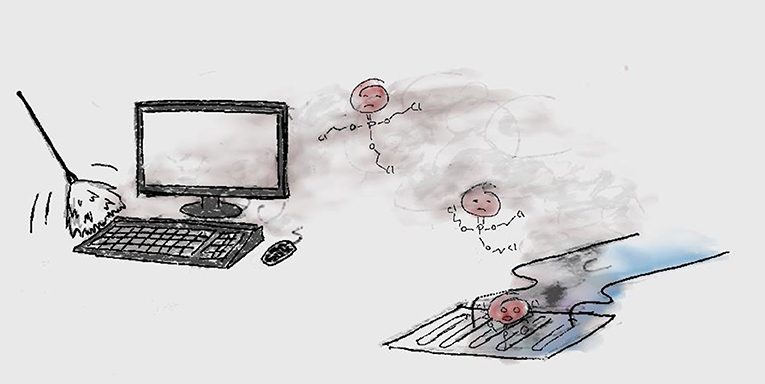
- Figure 1 - Flame retardants dusted off a computer can get into the environment through the air and rainwater (in this case through the sewers).
Because of these dangerous properties, some flame retardants are not allowed to be used anymore. But people were still worried that computers, couches, and other plastic things could burn. So, the chemical industry started using other types of flame retardants, called organophosphate esters, or OPEs. The OPEs were not supposed to be persistent or able to get all the way to the Arctic.
Because OPEs are supposed to be an environmentally friendly alternative for the old flame retardants, the industry started using them a lot. But even though they were believed to be more environmentally friendly, OPEs can still make humans and animals sick. Some of them are even suspected to cause cancer [6].
Moreover, when we analyzed air in the Canadian Arctic, we found OPEs. Even worse: we found more OPEs than the old flame retardants that OPEs were replacing [7].
How Do OPEs Get Into the Arctic?
We wondered how the OPEs could be getting to the Arctic. Tests had shown that most OPEs were not as persistent as the old flame retardants. Also, computer models had predicted that OPEs would not reach the Arctic [8].
But the measurements were very clear: OPEs are present in the Canadian Arctic.
So, we went back to the Arctic to take more samples to try to figure out how OPEs could make it there.
We took a ship that travels through the Canadian Arctic every summer. We used a pump from the ship to collect air and water samples. We collected samples many times over 7 years, to really make sure we did not miss anything and to find out if the OPEs were always present in the Canadian Arctic or just present sometimes [7].
What we found was that:
- OPEs were found in the Canadian Arctic every time we took samples.
- There are more OPEs in the Canadian Arctic than old flame retardants.
- OPEs seem to get to the Arctic through the air as well as the water [7] (Figure 2).
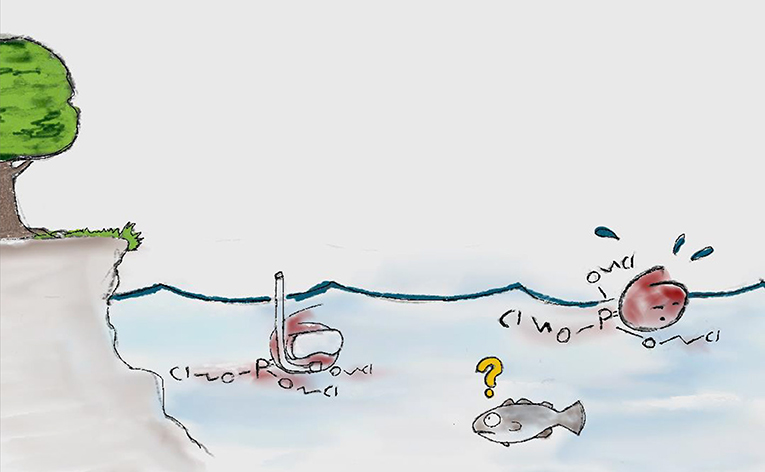
- Figure 2 - OPEs “swimming” (being transported through the water currents) from somewhere in the south (where they were made) toward the Arctic.
- In reality, OPEs are very tiny and there are a lot of them, but we had a hard time drawing that.
The idea that OPEs get to the Canadian Arctic through the water was a new result that nobody had really thought about before. This result might explain why so many OPEs are in the Arctic, because OPEs can degrade more quickly in the air than in water. So, if they are in the water, the OPEs could stay there long enough to get to the Arctic [7].
These results meant that OPEs are not a good alternative to the old flame retardants. Instead, just like the old flame retardants, OPEs are persistent enough to also get all the way to the Arctic (Figure 3). In some ways, OPEs are even worse than the old flame retardants, because we find a lot more of them in the Arctic [7].

- Figure 3 - OPEs in the Arctic, where they can get stuck in water, ice, and air.
Can Something Be Done About the Chemicals in the Arctic?
Yes, absolutely, something can be done.
The first step is that scientists, regulators, and industry need to check whether we really need all these flame retardants in the first place. Of course, nobody wants a computer or couch to burn easily, but some of the flame retardants may actually be worse than not having them because, if they burn, they produce a lot of toxic smoke. Also, other fire protection methods, such as sprinklers and smoke detectors, are much better at preventing fires than flame retardants [5]. So why not use more sprinkler systems and smoke detectors rather than flame retardants that can make animals and people sick?
If there is a case where we really need flame retardants, the industry that produces them should have to prove that the flame retardant they want to use is really less dangerous than the old ones that have been banned.
Very importantly, there are some things every one of us can do:
As adults, we can use our vote! Governments have many decisions to make and deciding which flame retardants are safe maybe is not at the top of the list. But we should all support governments that care about the environment and people’s health. This is our great power in a democracy.
Also, no matter what age we are, we can ask people in the shops where we buy plastic toys, phones, tablets, couches, or carpets whether these products have old flame retardants or OPEs in them, and if so, are the flame retardants really needed and do we really need that product? We can demand that the flame-retardant industry tell people if flame retardants are used and which kind are used in a product. This is important because, right now, when you and your parents go out to buy a new phone or couch, you cannot check to see if flame retardants are present in the products you want to buy. If more and more people ask for products without flame retardants, the shops, the industry, and the government will start thinking more about whether flame retardants are really needed in so many products.
Take this one step further and we can ask ourselves if we really need to buy so many new things all the time. Should not a phone, for example, last us longer than just 2 years?
Together, we can work toward limiting the use of dangerous chemicals like flame retardants, to protect the environment and human health.
Glossary
Degradation: ↑ Breakdown of chemicals in the environment. Degradation can happen because of bacteria, water, or even light.
Persistent Chemical: ↑ A chemical that does not break down easily in the environment. Some persistent chemicals can stay in the environment for tens and even hundreds of years.
Arctic: ↑ The high north of our earth, around the North Pole. The Arctic is not one country but several countries are part of the Arctic: Canada, Denmark (Greenland), Finland, Iceland, Norway, Sweden, Russia, and USA. The largest part of the Arctic is not on land but is covered by water—the Arctic Ocean.
Industry: ↑ All the companies together.
Flame Retardants: ↑ Chemicals that are used in a lot of plastic to make sure the plastic does not burn easily.
OPEs: ↑ A new type of flame retardant that was supposed to be an environmentally friendly alternative for the old flame retardants that were banned by the government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Sebastien Samson for the illustrations.
Original Source Article
↑Sühring, R., Diamond, M. L., Scheringer, M., Wong, F., Pucko, M., Stern, G., et al. 2016. Organophosphate esters in Canadian Arctic air: occurrence, levels and trends. Environ. Sci. Technol. 50:7409–15. doi: 10.1021/acs.est.6b00365
References
[1] ↑ Stockholm Convention. 2008. The New POPs Under the Stockholm Convention. Chatelaine: Secretariat of the Stockholm Convention. Available online at: http://chm.pops.int/Implementation/NewPOPs/TheNewPOPs/tabid/672/Default.aspx
[2] ↑ AMAP. 1998. AMAP Assessment Report: Arctic Pollution Issues. Oslo: Arctic Monitoring and Assessment Programme (AMAP).
[3] ↑ Scheringer, M. 2009. Long-range transport of organic chemicals in the environment. Environ. Toxicol. Chem. 28:677–90. doi: 10.1897/08-324R.1
[4] ↑ Rodgers, T. F. M., Truong, J. W., Jantunen, L. M., Helm, P. A., and Diamond, M. L. 2018. Organophosphate ester transport, fate, and emissions in Toronto, Canada, estimated using an updated multimedia urban model. Environ. Sci. Technol. 52:12465–74. doi: 10.1021/acs.est.8b02576
[5] ↑ Shaw, S. D., Blum, A., Weber, R., Kannan, K., Rich, D., Lucas, D., et al. 2010. Halongenated flame retardants: do the fire safety benefits justify the risks? Rev. Environ. Health 25:261–305. doi: 10.1515/reveh.2010.25.4.261
[6] ↑ Greaves, A. K., and Letcher, R. J. 2017. A review of organophosphate esters in the environment from biological effects to distribution and fate. Bull. Environ. Contam. Toxicol. 98:2–7. doi: 10.1007/s00128-016-1898-0
[7] ↑ Sühring, R., Diamond, M. L., Scheringer, M., Wong, F., Pucko, M., Stern, G., et al. 2016. Organophosphate esters in Canadian Arctic air: occurrence, levels and trends. Environ. Sci. Technol. 50:7409–15. doi: 10.1021/acs.est.6b00365
[8] ↑ Zhang, X., Sühring, R., Serodio, D., Bonnell, M., Sundin, N., and Diamond, M. L. 2016. Novel flame retardants: estimating the physical-chemical properties and environmental fate of 94 halogenated and organophosphate PBDE replacements. Chemosphere 144:2401–7. doi: 10.1016/j.chemosphere.2015.11.017