Abstract
Bones are the building blocks of our skeletons—they give our bodies structure and allow us to walk, dance, and pick things up. Healthy bones are strong, lightweight, hard, and even able to fix themselves when they get worn out or damaged. But if bones get sick, they can no longer repair themselves and they start to break down. One disease that makes bones sick is called osteoporosis and it can be very painful. Currently, doctors cannot cure osteoporosis. While there are medications to slow bone loss, there are no safe treatments to repair bone that is already damaged. Therefore, doctors and scientists are working together to find ways to prevent and even cure diseases like osteoporosis. One important challenge that needs to be addressed is: how can medicine be delivered to sick bones? In this article, we discuss how tiny carbon nanoparticles could help doctors deliver medicine to sick bones to repair them.
The Skeleton Is Made Up of Bones
The skeleton is the structure that gives shape to your body and allows you to stand and move. The adult skeleton is made of about 206 interconnected bones, in various shapes and sizes (see here for a Frontiers for Young Minds article on bones). For example, the longest bone in the body is called the femur. It is located in the upper leg, has a rod-like shape, and allows you to stand and run (Figure 1A). The smallest bone in the body is called the stapes. It is located inside the middle ear, has a padlock-like shape, and it allows you to hear sounds (Figure 1A). Despite these differences in shape, size, and function, all the bones in your body share several characteristics. All bones are lightweight, strong, and hard. Bones start forming before birth and continue to develop during a person’s teenage years and early adult life. Bones are constantly rebuilt to restore damaged and worn-out areas (Figures 1B, C). The best way to keep your bones healthy and strong is to follow a healthy diet and exercise regularly.
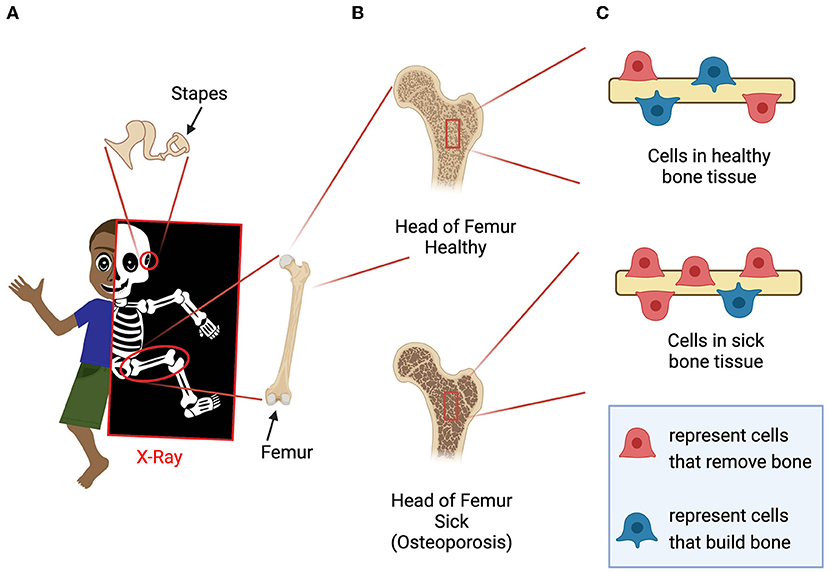
- Figure 1 - (A) The bones of the skeleton can be visualized using X-rays.
- The femur and the stapes are highlighted as examples. (B) Healthy people have strong bone tissue, but in people with diseases such as osteoporosis, bone tissue is weak. (C) Bone tissues are constantly being broken down and rebuilt by specialized cells. In people with osteoporosis, there are more cells breaking down bone than rebuilding it. This imbalance causes the bones to become brittle (Created with Adobe Illustrator and BioRender.com).
Sticks and Stones May Break My Bones
Bones are strong, but they can break because of accidents. Usually, when a bone breaks, doctors put the broken pieces together and cover the area with a cast to let the patient’s body repair the damage. If the patient has healthy bones, the healing process can take between 4 and 8 weeks. If the patient’s bones are sick, the healing process will take much longer or might never happen. One reason bones can be sick is because of disease. The most common disease that makes bones sick is osteoporosis, a type of bone disease that weakens bones. Osteoporosis mostly affects adults over 50 years old. As many as two out of ten adults have osteoporosis [1]. People with osteoporosis have fragile bones because the rebuilding process that restores damaged and worn-out areas is not working properly [1, 2]. In these people, the processes that get rid of damaged bone are taking place at a normal rate, but the processes that build new bone have slowed down (Figures 1B, C) [2]. Over time, the bones become weak, brittle, and can easily break. If someone has a very serious case of osteoporosis, they might even break a bone sneezing! Currently, doctors have no treatments to cure osteoporosis. While doctors can prescribe medicine to prevent the bones from becoming more fragile over time, they do not have medications to help a patient’s body rebuild damaged bone [2]. Together with scientists, doctors are trying to find new treatments to cure or even prevent osteoporosis and other bone diseases.
Diagnosing and Treating Bone Disease
To find where a bone is broken, doctors can use a variety of imaging machines. For example, X-ray machines can take pictures of the bones inside your body. While these machines can also be used to detect bones that are sick, doctors do not recommend frequent X-ray imaging because it can be dangerous. Therefore, people often do not know that their bones are sick until they break a bone and have to get an X-ray. By then, the bones have already weakened because the body’s process of removing damaged bone has continued without new, healthy bone being added [2].
Once doctors diagnose osteoporosis, they prescribe medicines that prevent further bone breakdown, stopping the bones from becoming even weaker [2]. These medications do not help the patients’ bodies make new healthy bone tissue, meaning their bones will remain weak, painful, and at risk of breaking for the rest of their lives. To strengthen sick bones, doctors would need to prescribe medicine that encourages the bone cells to make more of themselves. Doctors do not prescribe these medications because these medicines encourage all the cells in the body—not just the bone cells—to make more of themselves and grow. This can potentially lead to cancer—see this Frontiers for Young Minds article to learn more about it. If scientists could find a way to deliver these medications only to bones, then it would be safe to give these bone-healing medications to people with osteoporosis.
Using Zebrafish to Study Drug Delivery to Bones
Before a medicine is given to a patient, it must go through many safety tests. For example, doctors and scientists must know how much of the medication to give to the patient, how long the patient should take the medication, and whether there are side effects. Side effects are an important medical concern because a medicine that is meant to help in one tissue of the body can cause harm in a different tissue. For this reason, doctors must make sure a medicine does not have side effects and primarily targets the sick tissue.
Scientists can answer these questions by testing medicines in animals. They do this carefully, under the supervision of other scientists and community members, to make sure that the animals are treated respectfully and do not suffer pain. In the past, medications have been tested on mammals like mice, but recently other animals have become popular because they have advantages that allow scientists to more easily explore the activity and delivery of medications.
One animal that has become popular for testing medications in the last 20 years is the zebrafish. Zebrafish are small fish with black and silver stripes, like zebras, and are about the size of a human thumb. Originally from India, zebrafish can now be found in laboratories all around the world, and even at your local pet store [3]. One reason zebrafish have become popular is that scientists discovered a type of transparent zebrafish that do not have black and silver stripes. These see-through zebrafish allow scientists to use microscopes instead of X-ray machines to see their skeletons.
Could Carbon Nanoparticles (C-Dots) Deliver Medicine to Bones?
Very often, scientists from separate fields work together to solve difficult problems. Chemists are good at making new materials, and biologists are good at testing materials in animals to see if and how doctors could use those materials to help sick people. In the last 10 years, chemists and biologists have been interested in finding new compounds that can deliver medications to specific tissues. One of these new compounds are carbon nanoparticles, or C-dots for short. C-dots are so small that 10,000 of them could fit inside a grain of salt (Figure 2). Additionally, C-dots are peculiar in that they can “glow in the dark” when observed under a special fluorescent light, which makes them easy to see and follow in animals, especially in animals that are see-through (Figure 3). To discover whether C-dots could be useful to medical doctors, a group of chemists asked our lab to test these new compounds in zebrafish [4].
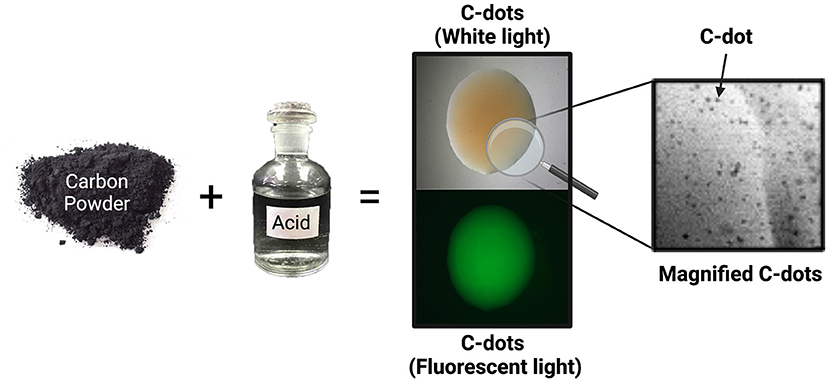
- Figure 2 - To make carbon nanoparticles (C-dots) in the laboratory, chemists mix carbon powder and acids for 16 hours.
- C-dots are then purified from the acid. C-dots look brown under regular white light. Under fluorescent light—a special light that can make some materials glow—C-dots appear bright green. C-dots are so tiny that 10,000 C-dots can fit inside a grain of table salt. Because they are so small, we can only observe C-dots using powerful microscopes (Created in BioRender.com).
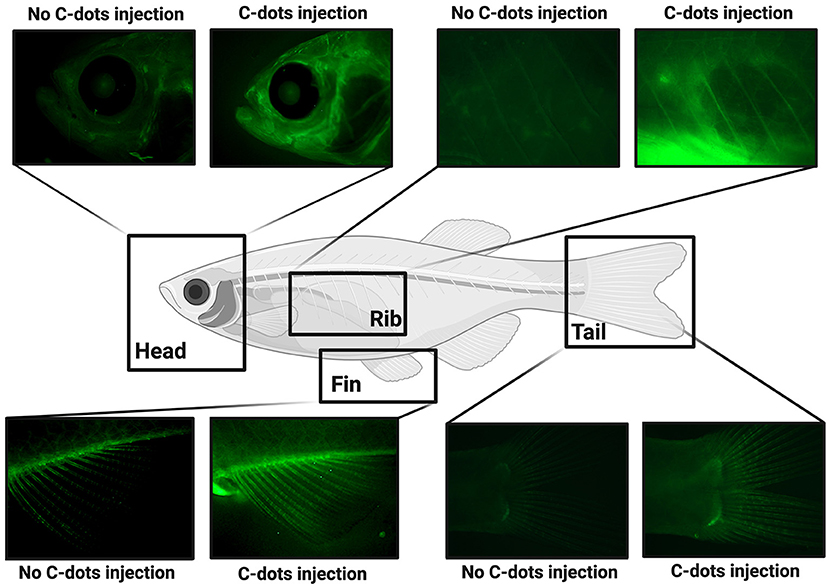
- Figure 3 - After C-dots are injected into transparent zebrafish, their bones glow under fluorescent light (Created in BioRender.com).
When our lab injected the C-dots into transparent adult zebrafish, we discovered that their skeletons glowed under fluorescent light (Figure 3) [4]. To safely deliver C-dots to the fish, we first put the fish to sleep using anesthesia, just like when humans get surgery, and then carefully gave the fish a shot containing C-dots. After the fish recovered in their tanks for several days, we anesthetized them again and photographed them under fluorescent light using a microscope. Because the zebrafish were transparent, we could easily see all the places where the C-dots remained in the fish. Excitingly, we saw green C-dots attached to the fish skeleton, but not other tissues, suggesting that the C-dots were especially attracted to bones. Other than having glowing bones, these injected fish were as healthy and active as fish that did not receive the C-dots. Together these observations suggest that C-dots specifically bind to bone tissue and do not interfere with the wellbeing of fish.
Conclusion
Finding a compound that collects only on bone tissue could provide doctors with a novel and safe way to treat osteoporosis. For example, if a medication that promotes tissue growth and repair was attached to C-dots, then C-dots could deliver it straight to bones. This would reduce the potentially dangerous side effects caused by delivering these medications to all the tissues in the body. Another advantage of delivering medicine directly to injured or sick bone is that it would reduce the amount of medication needed, because the medicine would be concentrated in the place where it needs to work. Our work is the first step in identifying C-dots as compounds that can be used to deliver medicines directly to bones. Further work is needed to show that C-dots are safe and reliable, and that they don’t interfere with the activity of bone-healing medications.
Glossary
Osteoporosis: ↑ A type of bone disease that weakens bones. Osteoporosis means “porous bones” (pores are tiny holes), because bones that are sick with osteoporosis look like honeycombs under the microscope.
Side Effects: ↑ A unwanted consequence of taking a drug or medical treatment that was not expected to happen (ex. nausea, dizziness, or even developing another disease like cancer).
Compound: ↑ A chemical substance that is composed of many of the same molecules, each composed of two or more different atoms.
Carbon Nanoparticles: ↑ Tiny particles made of carbon atoms that are < 0.0001 mm across.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank Esmail Miyanji for helping collect information for the original publication and Zach Perkins and Quan Chau for providing constructive criticism. Special thanks to Phinn Hilliker for reviewing the manuscript prior to publication. We also want to thank the organizations that supported this research: the National Science Foundation (DMR 1809419), the National Institute of Health (NIAMS R21AR072226) and the University of Richmond School of Arts and Sciences.
Original Source Article
↑ DuMez, R., Miyanji, E. H., Corado-Santiago, L., Barrameda, B., Zhou, Y., Hettiarachchi, S. D., et al. 2021. In vivo characterization of carbon dots-bone interactions: toward the development of bone-specific nanocarriers for drug delivery. Drug Deliv. 28:1281–9. doi: 10.1080/10717544.2021.1938753
References
[1] ↑ Wright, N. C., Looker, A. C., Saag, K. G., Curtis, J. R., Delzell, E. S., Randall, S., et al. 2014. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 29:2520. doi: 10.1002/jbmr.2269
[2] ↑ Tu, K. N., Lie, J. D., Wan, C. K. V., Cameron, M., Austel, A. G., Nguyen, J. K., et al. 2018. Osteoporosis: a review of treatment options. P T. 43:92.
[3] ↑ Tonelli, F., Bek, J. W., Besio, R., De Clercq, A., Leoni, L., Salmon, P., et al. 2020. Zebrafish: a resourceful vertebrate model to investigate skeletal disorders. Front. Endocrinol. 11:489. doi: 10.3389/fendo.2020.00489
[4] ↑ DuMez, R., Miyanji, E. H., Corado-Santiago, L., Barrameda, B., Zhou, Y., Hettiarachchi, S. D., et al. 2021. In vivo characterization of carbon dots-bone interactions: towards the development of bone-specific nanocarriers for drug delivery. Drug Deliv. 28:1281. doi: 10.1080/10717544.2021.1938753