Abstract
When you cook, do you know why it smells so good (or so bad) in the house? It is all about chemistry. All over the world, cooking is based on tradition and love, but it is the chemistry of the foods that allows recipes to succeed or not. Among the chemical reactions that occur in cooking food, the Maillard reaction, which occurs between amino acids and sugars, is the most familiar. This is actually a complex series of reactions that lead to the formation of aromas, tastes, and colors as food cooks. In this article, we will discuss the most important stages of the Maillard reaction that happens when we bake, barbecue, or otherwise cook foods. Once the Maillard reaction is complete, you just have to say, “enjoy the meal!”
Foods contain many compounds, including proteins, sugars, and lipids, which are called macronutrients; and vitamins and minerals, which are called micronutrients. Both macro- and micronutrients are important parts of our diets and contribute to human wellbeing. The specific combinations of these compounds give rise to the unique flavors we taste. But cooking plays a role, too! Freshly baked breads and pastries, barbecued meats, and golden-brown potatoes all smell very good. What do these delicious foods have in common? A special chemical reaction called the Maillard reaction happens during the cooking of these foods. The Maillard reaction could be called the queen of cooking reactions, and you will soon understand why.
Basics of Chemistry
First, what is a chemical reaction? A chemical reaction occurs when we put two or more things together and produce something new. In the Maillard reaction, the key players are sugars and amino acids (Figure 1), which are present in many foods. Amino acids are the building blocks for making proteins. In the Maillard reaction, a specific part of the sugars called the carbonyl group reacts with a part of amino acids called the amino group, producing water and a new compound that is created by the joining of the two molecules.
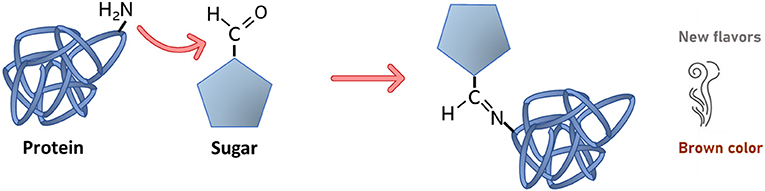
- Figure 1 - Maillard reaction between a specific part of the sugar, called the carbonyl group, and amino group of a protein.
The Maillard Reaction Is Responsible for How Foods Taste and Smell
The Maillard reaction is responsible for the taste, aroma, and color of cooked foods. This reaction happens efficiently at high temperatures (over 140°C) and the result of the reaction depends on how long the reaction goes on. There are three stages in the Maillard reaction: the early stage, the advanced stage, and the final stage. During the early stage, there is no visible effect on the food. But this initial phase is when sugars and amino acids bond to each other to form key compounds that are then used in the next two stages.
Flavor Is Set During the Advanced Stage
The advanced stage is responsible for producing the flavors in cooked foods—both the aromas and the tastes. Flavors change depending on the types and combinations of amino acids and sugars in the food, as well as on the temperature and length of cooking time. Aromas, formed during the Maillard reaction, are mainly due to sugars turning into various kinds of compounds, including pyrazines. Due to their small size, these compounds are volatile, which means they are in a gaseous state at room temperature and thus can be perceived by the sense of smell (Figure 2). Pyrazines are important components in the flavor of cooked, roasted, and toasted foods. They give a distinctive aroma to beef products, toasted barley, peanuts, hazelnuts, popcorn, and cocoa products such as chocolate, coffee, and potato products.
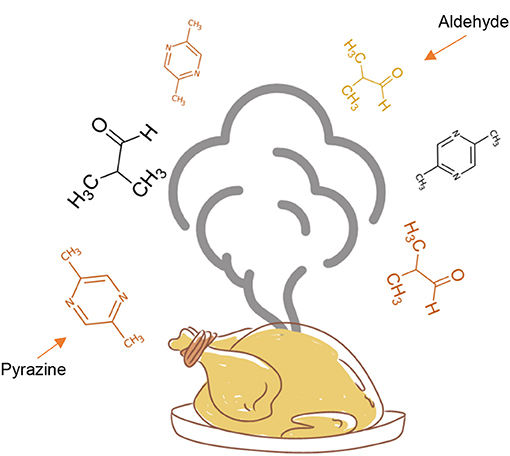
- Figure 2 - When chicken is roasted, the advanced stage of the Maillard reaction produces volatile compounds including aldehydes and pyrazines, which are released into the air and reach our noses, allowing us to smell the aroma of the cooked food.
Other molecules produced during the advanced stage of the Maillard reaction are also very important for giving odors to cooked food. Maillard reaction products (MRPs) include substances called Amadori compounds, Heyns compounds, aldehydes, and pyrazines we discussed earlier. These substances contain sulfur, which comes from certain amino acids. When these amino acids participate in the reaction with sugars, they create new compounds with meat-like, fried chicken-like, and French fry-like aromas [1].
The Maillard Reaction Makes Food Look Good
The final stage of the Maillard reaction creates large molecules called melanoidins. In addition to the pigments naturally found in foods, a food’s color can be modified by a series of chemical reactions that lead to darkening. The compounds made during these chemical reactions create the brown crusts of baked goods and the charred streaks on grilled meat. The darkening of dried fruits, cocoa, and coffee beans during roasting also involves melanoidin formation. The presence of certain sugars and proteins in cookie dough leads to cookies with a golden yellow-brown color, whereas the absence of those sugars and proteins can lead to cookies with a very dark color. Food is often judged by its looks. Which cookies would you rather buy in the bakery—golden yellow-brown ones, or dark-looking ones? Melanoidin and other MRPs generated during cooking are responsible for the appealing look of cookies and other cooked foods.
The Maillard Reaction Can Produce Various Tastes
Compounds involved in the Maillard reaction also include some very important taste-active compounds [2]. MRPs can generate specific different tastes such as umami, a typical taste of broths and roasts, and kokumi, a Japanese word to describe a taste similar to garlic and onions. These taste-active compounds can give rise to unique tastes depending on the age of the food product. For example, fresh and aged cheeses have different tastes when treated with heat.
The temperature of cooking can also influence the taste of food. For example, if you want your chicken to have a meaty, kokumi aroma, you have to cook it at a temperature higher than 100°C for a short time. Alternatively, if you would like your chicken to have a broth-like taste, cook it at a low temperature for a long time [3]. An umami flavor can also be found in slow-cooked Italian tomato sauce.
MRPs can be Used for Food Preservation
Some MRPs from the advanced and final stages are also important for preserving food. In fact, MRPs such as melanoidins are often added to foods by the food industry as preservatives, so that the foods can be stored for a long time. For example, potato chips, biscuits, and cookies are preserved by melanoidins. Without these preservatives, mold would grow on the foods, and they would spoil—and start to taste bad—very quickly.
The Maillard Reaction can Produce Toxic Compounds
Unfortunately, in addition to favorable compounds that give rise to delicious odors, flavors, and appealing colors, some toxic (or at least undesirable) compounds can also be produced by the Maillard reaction—especially when foods are cooked for a long time. Acrylamide is one such toxic compound—it can modify the DNA of cells and lead to cancer. Acrylamide can be found in foods containing sugars and amino acids, which are charred or cooked at extremely high temperatures.
Enjoying Good Food can Lead to Wellbeing
The MRPs generated through the Maillard reaction can have positive effects on the body and mind. The scents generated by the reactions can lead to feelings ranging from calm and relaxed to refreshed and vigorous. Additionally, these smells reduce blood pressure, tension, restless, and fatigue, and they decrease negative moods [3]. This could be part of the reason cooking brings people together and puts everyone in a good mood—cooking is a combination of chemistry and love! Food helps to bring people together, to smile, and celebrate. So, at the end of the Maillard reaction, you just have to say, “enjoy your meal,” laugh, and eat with your family and friends.
Glossary
Maillard Reaction: ↑ Reaction between sugars and amino acids. It occurs during the cooking of food.
Amino Acids: ↑ Molecules that are the building blocks for making proteins.
Pyrazine: ↑ Small organic molecule formed from carbon and nitrogen atoms; it is characterized by an aromatic smell.
Volatile: ↑ Molecules that are in a gas state at room temperature due to their small size. Because of this, we can smell them.
Maillard Reaction Products (MRPs): ↑ Amadori compounds, Heyns compounds, aldehydes, and pyrazines. Molecules are produced at different stages of the Maillard reaction.
Melanoidins: ↑ Dark brown compounds that are formed when sugars and amino acids combine (through the Maillard Reaction) at high temperatures.
Umami: ↑ Typical flavor of broths and roasts.
Kokumi: ↑ Word to describe a taste similar to garlic and onions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Chuyen, N. V. 1998. Process-Induced Chemical Changes in Food. Tokyo: Maillard Reaction and Food Processing Plenum Press.
[2] ↑ Zamora, R., and Hidalgo, F. J. 2005. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 45:49–59. doi: 10.1080/10408690590900117
[3] ↑ Arihara, K., Zhou, L., and Ohata, M. 2017. Bioactive properties of maillard reaction products generated from food protein-derived peptides. Adv. Food Nutr. Res. 81:161–85. doi: 10.1016/bs.afnr.2016.11.005

