Abstract
Can you imagine a world in which doctors prescribe food when we are sick? Traditionally, doctors have prescribed medications to treat sickness. Most of these medications, known as pharmaceuticals, were developed by researchers. Pharmaceuticals are made to interact with molecules associated with specific diseases, to reduce patients’ symptoms. Recent studies have shown that natural compounds found in foods can alleviate illnesses. In contrast to pharmaceuticals, we can take in these compounds, called nutraceuticals, through the foods we eat every day. Nutraceuticals have been studied in various organisms, and their effects on the bacteria that live in the digestive system have also been examined. Studies in fruit flies have shown that nutraceuticals can be beneficial for treating some brain diseases. This article will describe promising nutraceuticals that could be used to treat Alzheimer’s disease, which affects many older people worldwide.
When Forgetting Becomes a Problem
How many times have you forgotten the name of a new friend you met at a party? Or how many times have you arrived at school in the morning and suddenly remembered that you had a test that day? This is nothing to worry about; we are all forgetful sometimes! But when people get old, forgetting simple information and tasks could be a sign of a neurodegenerative disease. A neurodegenerative disease is an irreversible, worsening brain disease that slowly destroys brain cells, which are called neurons. Alzheimer’s disease (AD) is a well-known neurodegenerative disease that affects over 36 million people worldwide, mostly over the age of 65 [1, 2]. The first neurons affected in AD are those responsible for memory and thinking skills. As AD advances, patients lose the ability to carry out normal daily tasks, they experience behavioral changes, and they slowly lose the ability to eat, drink, and communicate. It is important to note that AD is different from normal aging. In AD patients, the loss of neurons and the decline in memory and other skills happen more rapidly than in aging people without AD.
Stages of Alzheimer’s Disease
AD is a progressive disease, meaning that it gets worse over time. AD can be classified into three main stages—early, middle, and late—each characterized by specific symptoms (Figure 1) [1].
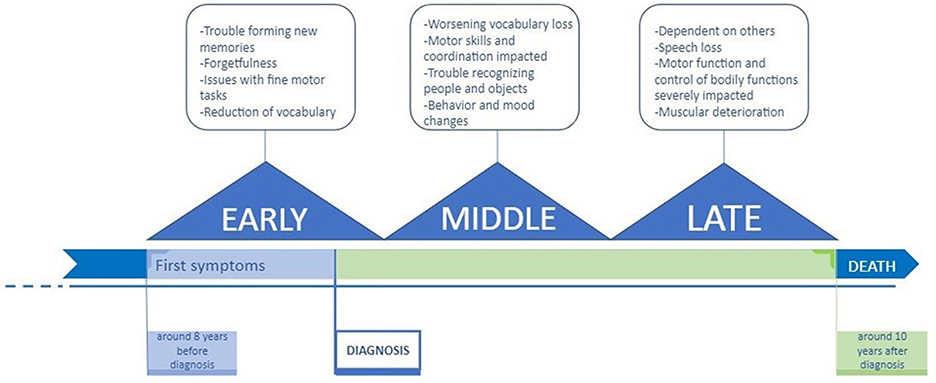
- Figure 1 - Three stages of Alzheimer’s disease.
- AD generally proceeds through three stages: early, middle, and late. Each stage is characterized by specific symptoms. However, because the earliest symptoms are often mistaken as simple signs of aging, a diagnosis of AD may take up to eight years after the first symptoms appear [1]. Patients can live ~10 years after diagnosis, during which time symptoms worsen.
The early stage is often improperly diagnosed as “old age.” Generally, patients are diagnosed with early-stage AD around age 65 [2]. Patients in the early stage of AD can have difficulty with simple tasks that involve movement, such as buttoning shirts or opening jars. People who do not have AD can complete such tasks with little effort. In addition, AD patients typically have a reduced vocabulary, difficulty with learning, and problems remembering new information like new names or directions [1].
During the middle stage, patients typically start to have difficulty living independently. Declining motor skills and coordination, which affect walking, can increase the possibility of injuries. Patients begin having trouble with long-term memory, preventing them from recognizing familiar faces, including those of their loved ones. In this stage, patients start to experience unusual behavior and mood shifts. At this stage, patients may need home care or assisted living care to live their daily lives [1].
In the last stage of AD, the symptoms are the most severe. During this final stage, patients require round-the-clock care. Individuals tend to lose the ability to care for themselves, including their most basic needs such as bathing, moving, and eating. At this stage, patients also lose the ability to hold conversations or to communicate their wants, needs, and feelings [1]. Eventually, patients require end-of-life care, so that they can live out their final days as comfortably as possible.
What Causes Alzheimer’s Disease?
If you had to guess how many cells your brain contains, what would your guess be? Ten thousand, one hundred thousand, or even one million? Our brains actually contain around 100 billion neurons! Neurons send and receive messages, allowing quick communication between the brain and other parts of the body. In the brains of AD patients, these connections are interrupted, but scientists do not fully understand why. Research has identified three possible causes of AD [1]. When a person has AD, pieces of a protein called amyloid beta accumulate between neurons, forming structures called amyloid-beta plaques outside of the cells. Another unusual protein called tau builds up inside neurons, forming dense tangles. The amyloid-beta plaques and tangles block neurons from sending and receiving signals, leading to neuron death. As a result, certain parts of the brain tend to shrink in size because of the death of many neurons, causing the symptoms described above.
Studying Alzheimer’s Disease in Fruit Flies
While it would seem that fruit flies and humans are incredibly different, in reality they are about 75% similar when it comes to disease-related genes [3]. The gene coding for a protein called amyloid precursor protein (APP), which is one of the proteins responsible for AD, is also present in fruit flies, where it is known as APP-like protein (APPL) [1]. Human APP can be inserted into flies, which are easy to perform experiments on, using modern genetic tools.
Some AD experiments performed in fruit flies involved producing mutated human APP proteins in fly brains. The mutations chosen had been found in AD patients [1]. One such experiment showed that flies with a mutated APP had abnormal sleep patterns, which is also common among early-stage AD patients [1]. Another experiment showed that a mutated APP disrupted the normal “house cleaning” functions within cells, causing the buildup of certain unnecessary molecules [1]. Researchers also created flies in which APPL was absent or present at reduced levels, and saw that the learning and memory skills of these flies were negatively affected [1].
AD-related symptoms can be assessed in flies by looking for changes in the shapes of their neurons [4]. Symptoms can also be assessed by looking at fly behavior, for example by using a climbing test that measures the ability of flies to move [5]. In summary, because they are easy to study in the lab and show similarities to humans in terms of symptoms and the proteins involved (Figure 2), fruit flies are extremely useful for understanding the mechanisms of AD.
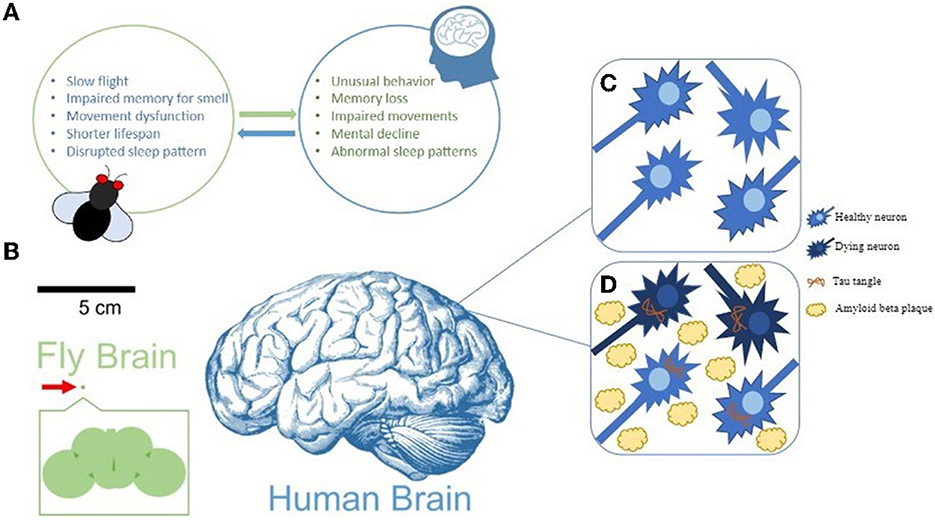
- Figure 2 - Signs of AD in humans and fruit flies.
- (A) Fruit fly models of AD show symptoms similar to those observed in humans. (B) The fruit fly brain is about 250 μm in size, compared to the average human brain, which is about 15 cm long. (C,D) In AD, healthy neurons die due to amyloid beta plaques between cells and tau tangles within cells.
Pharmaceuticals and Nutraceuticals in AD
Unfortunately, there are currently no cures for AD, but researchers are trying to treat the disease in a variety of ways. The main treatment approach involves pharmaceutical compounds (drugs) that interact with various molecules known to play a role in AD. Before they can be given to patients, these pharmaceuticals must first go through an approval process to assess how well they work and whether they are safe. Many pharmaceuticals to treat AD have been developed by large companies in recent years; however, most of them have failed the approval process due to severe side effects [1]. This has left researchers looking for alternative treatments for AD.
Luckily, another promising class of compounds, called nutraceuticals, might help with AD symptoms. Although the terms “nutraceuticals” and “pharmaceuticals” sound similar, nutraceuticals come from foods that people eat daily. Nutraceuticals are naturally occurring food chemicals that may have medicinal properties [1]. Examples of nutraceuticals that may reduce AD symptoms include gallic acid, anthocyanin, cinnamaldehyde, curcumin, and silybin (Figure 3) [1]. Even though you may have not heard their names before, these chemicals are present in foods that you probably know very well. For example, gallic acid is found in fruits like strawberries, bananas, and grapes. Gallic acid has been shown to reduce the levels of toxic molecules in the body and to reduce the accumulation of amyloid beta plaques [1]. Anthocyanin is another promising nutraceutical. It produces the blue/purple color in blueberries, mulberries, and other fruits and vegetables. Anthocyanin also lowers toxic molecule levels and is beneficial for the digestive system [1]. Finally, cinnamaldehyde (found in cinnamon), curcumin (found in turmeric and curry spices), and silybin (found in milk thistle used in herbal tea) are all common household compounds shown to alleviate some AD-associated symptoms based on observations in fruit flies [1].
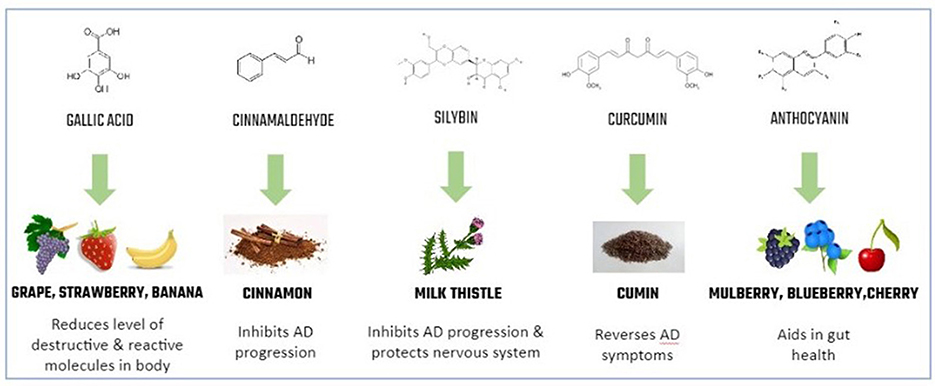
- Figure 3 - Common nutraceuticals and their benefits.
- Nutraceuticals are chemicals with medicinal benefits that are naturally found in foods.
In addition to directly improving some of the symptoms of AD, some nutraceuticals have also been shown to act indirectly, through impacting the bacteria in the digestive system [1]. A wide variety of bacteria live in the human digestive system; together they are called the gut microbiome. The gut microbiome influences many parts of the body. This idea of bacteria in the gut helping the brain may sound bizarre, but several studies have shown that the gut microbiome influences brain function via what is called the brain-gut-axis [1]. Scientists have closely examined the effects of the microbiome on AD symptoms in many organisms, and they have found some common features, including effects on the immune system, inflammation, and brain function [1].
Some fermented foods contain live microorganisms called probiotics that are known to produce beneficial effects when eaten. Examples of probiotic foods include yogurt, kefir, kombucha, and sauerkraut. Researchers are studying how organisms can benefit from probiotics. For example, one probiotic treatment included three bacterial strains often found in fermented foods, paired with an herbal nutrient-rich compound that promotes the growth of beneficial microorganisms (such compounds are called pre-biotics) [1]. This treatment helped flies to live longer, improved their mobility, and reduced the accumulation of amyloid beta plaques in their brains [1]. Although these results are promising, further research is needed to establish the relationship between nutraceuticals, the gut microbiome, and AD.
Conclusion
Alzheimer’s disease is far from being cured. However, scientists are working hard to develop safe and effective ways to target factors that are important in AD and to lessen AD symptoms. Nutraceuticals—substances that are found in many of the foods we eat daily—are a promising alternative to pharmaceutical AD treatments which, so far, have not proven very effective. Beyond nutraceuticals, recent advances in molecular biology and medicine have opened up additional avenues for potential treatments. This combination of advancements, together with creative new methods that will certainly be invented, make the potential for understanding and effectively treating AD higher than ever. Hopefully, future discoveries in AD research will positively impact the lives of countless elderly individuals and their families.
Glossary
Neuro-Degenerative Disease: ↑ A type of disease in which cells of the brain or spinal cord stop working or die.
Neuron: ↑ Type of cell able to send electrical and chemical signals that are important in brain function and memory.
Amyloid-Beta Plaque: ↑ Buildup of the Amyloid-beta protein fragment happening in the brains of AD patients.
Pharmaceuticals: ↑ Substances made by a drug company for medical purposes.
Nutraceuticals: ↑ Ingredients found in the food we eat that could potentially be used to treat disease.
Gut Microbiome: ↑ Collection of microorganisms found in the gastrointestinal tract.
Probiotics: ↑ Supplements containing live bacteria or yeast that helps our gut microbiome.
Pre-Biotics: ↑ Foods that stimulate the growth or activity of beneficial microorganisms in the gut.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank members of the Vonhoff lab for helpful comments on the manuscript. Thanks to the UMBC Natural Sciences Pre-Professoriate Fellowship for support to FV. AC and CP were supported by the LSAMP Program at UMBC, which was funded through an award from the National Science Foundation (Award #1002566). CP was also supported by the Undergraduate Research Award program at UMBC, which was funded by the Division of Undergraduate Academic Affairs, by the Meyerhoff Scholars Program, by a grant to UMBC from the Howard Hughes Medical Institute through the HHMI Adaptation Project, and by the U-RISE Program at UMBC. URISE at UMBC was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIGMS/NIH) under Award T34GM136497.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
[1] ↑ Jalali, D., Guevarra, J. A., Martinez, L., Hung, L., and Vonhoff, F. J. 2021. Nutraceutical and probiotic approaches to examine molecular interactions of the amyloid precursor protein APP in Drosophila models of Alzheimer’s disease. Int. J. Mol. Sci. 22:7022. doi: 10.3390/ijms22137022
[2] ↑ Alzheimer’s disease facts and figures. Alzheimers Dement. (2020) 16:391–460. doi: 10.1002/alz.12068
[3] ↑ Tian, Y., Zhang, Z. C., and Han, J. 2017. Drosophila. studies on autism spectrum disorders. Neurosci. Bull. 33:737–46. doi: 10.1007/s12264-017-0166-6
[4] ↑ Sunderhaus, E. R., and Kretzschmar, D. 2016. Mass histology to quantify neurodegeneration in Drosophila. J. Vis. Exp. 15:54809. doi: 10.3791/54809
[5] ↑ Madabattula, S. T., Strautman, J. C., Bysice, A. M., O’Sullivan, J. A., Androschuk, A., Rosenfelt, C., et al. 2015. Quantitative analysis of climbing defects in a Drosophila model of neurodegenerative disorders. J. Vis. Exp. 100:e52741. doi: 10.3791/52741

