Abstract
Burning fossil fuels for energy produces greenhouse gases. Greenhouse gases cause climate change, which harms the environment and all living things. Hydrogen fuel can be used as an energy source instead of fossil fuels. We can produce hydrogen using two natural resources: sunlight and water. Hydrogen fuel is renewable, meaning it will not run out, and environmentally friendly because no greenhouse gases are produced. Materials called metal-organic frameworks (MOFs) can speed up the reaction that produces hydrogen gas. With the help of sunlight, MOFs can break apart water molecules to produce hydrogen gas. We can also break down pollutants if we use dirty water in this reaction. Using MOFs, we can purify water and produce hydrogen gas at the same time! Combining MOFs with sunlight can help solve two global problems: climate change and water pollution.
Climate Change and Its Challenges
In the United States, 59% of the total energy we use comes from fossil fuels [1]. Do you ride in a car or bus? Do you turn on the lights or a TV in your home? Every time you turn on the lights or ride in a car, you are using fossil fuels! Fossil fuels come from ancient plants and animals that lived millions of years ago. Coal, oil, and natural gas are all examples of fossil fuels. Fossil fuels must be burned to release the energy they hold. But burning fossil fuels releases greenhouse gases, which lead to climate change. When sunlight hits Earth, Earth’s surface absorbs the heat. Then, Earth releases the heat back to the atmosphere and outer space. Greenhouse gases in our atmosphere absorb this heat and prevent it from escaping to outer space. The heat gets trapped around our planet and acts like a heating blanket [2]. Too much greenhouse gas in our atmosphere is like piling more blankets on the Earth. These blankets cause Earth to heat up very quickly. Because of these extra blankets, global temperatures are increasing more quickly than ever.
Climate change is bad for our environment in multiple ways. Melting glaciers and severe droughts are some effects of climate change. We see the most noticeable results of climate change in wildlife. The ocean temperature and acidity are rapidly changing because we are releasing so much greenhouse gas. Fish and coral are sensitive to changes in their ocean habitats. Many ocean plants and animals cannot survive in a warmer and more acidic environment. Scientists predict that the rise in ocean temperature could kill more than 70% of all coral reefs [3]. This loss represents a small fraction of the total damage that climate change does to ecosystems. We must preserve Earth’s ecosystems because we learn and benefit from their beauty and complexity. We also need these ecosystems to feed humanity and provide essential resources.
All living things need water to survive. Climate change and environmental pollution make it harder for people to find clean water. It is estimated that 1 in 4 people worldwide does not have clean drinking water [4]. Sewage, factory waste, and oil pollute the water we drink. Drinking or using dirty water can make people and wildlife very sick. Finding new ways to clean polluted water is essential because we need clean water to survive and stay healthy.
A Promising Solution
Have you ever felt the warmth of the sun on your skin during a cloudless day? The sunlight hitting your skin makes you feel warm because it contains energy! We can use this energy from sunlight instead of burning fossil fuels. One way to capture the sun’s energy is to use a material that can absorb light. Then we can use the energy in sunlight to break apart water molecules! Breaking apart water molecules makes hydrogen gas (Figure 1A). Hydrogen gas can be used to power our lights, vehicles, and electronic devices. If we use dirty water, we can make hydrogen and purify water at the same time [5]! For example, we could use this process to break down antibiotics, pesticides, or dyes found in polluted water.
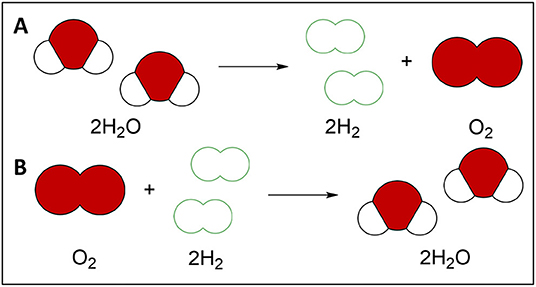
- Figure 1 - (A) Breaking apart water (H2O) forms hydrogen (H2) and oxygen gas (O2).
- (B) The combination of hydrogen (H2) with oxygen (O2) causes hydrogen to burn, releasing energy in the form of heat. Burning hydrogen gas for energy produces water (H2O).
When we burn hydrogen gas for its energy, we produce water and no greenhouse gases (Figure 1B). That means hydrogen fuel can power the planet without destroying its ecosystems! Hydrogen fuel is also more energy-dense than any fossil fuel. High energy density means more energy is packed into hydrogen fuel than into fossil fuel. Hydrogen fuel formed by water splitting is renewable, which means we will never run out. There is still much work and research to be done before our cars and buildings run on hydrogen fuel. But knowing we could have clean hydrogen fuel available is an essential step in moving the world away from fossil fuels. We can break apart pollutants in water to make hydrogen fuel to provide energy and clean water to billions of people!
What Materials Can We Use to Make H2?
To make hydrogen fuel using the sun’s energy, we need a material that functions as a catalyst. A catalyst assists and speeds up a chemical reaction. One type of catalyst, called a photocatalyst, borrows energy from sunlight to speed up chemical reactions. We can use photocatalysts to convert sunlight to hydrogen fuel while at the same time purifying water. Materials called metal-organic frameworks (MOFs) can be used as photocatalysts for this process [5]. These materials are made of metal parts and non-metal parts, called ligands. Like building with Legos, these parts connect to form a 3-dimensional framework on the atomic scale (Figure 2A). By using various ligands and metals, scientists can create different MOFs that have the properties they desire. The MOF framework has pores (small holes) in it, similar to a sponge. These pores allow the MOF to adsorb pollutants and water. Like table salt, MOFs look like a powder but are tiny crystals that you can see with a microscope.
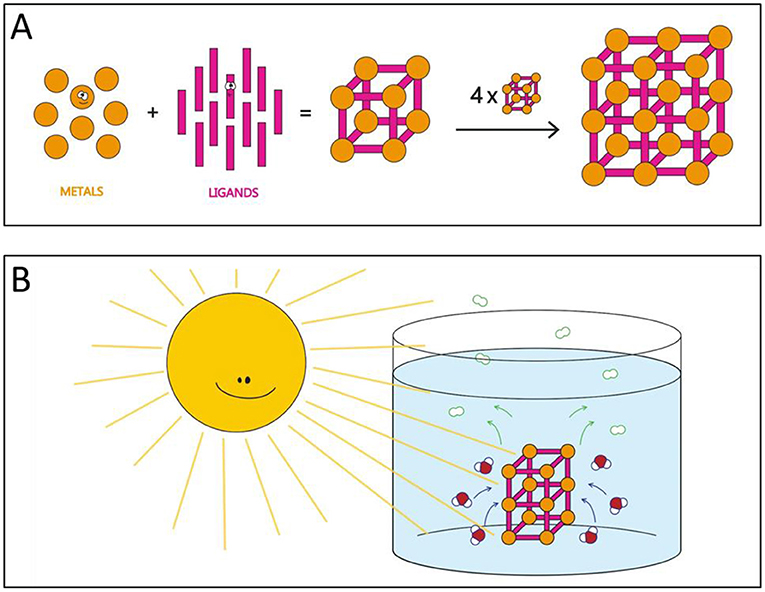
- Figure 2 - (A) MOFs are created by combining metals (orange) with organic ligands (pink) to form a framework (center).
- When combining multiple frameworks (like building with Legos), the framework grows in 3 dimensions to form a porous material (right). (B) Our MOF (pink and orange framework) in water (red and white molecules). Because our MOF is porous, water can move through its channels. When energy from the sun hits our MOF, it can split water and form hydrogen gas (white molecules outlined in green).
We can place the MOF in polluted water and expose the mixture to direct sunlight to purify dirty water and make hydrogen fuel (Figure 2B). The amount of hydrogen produced and amount of pollutants degraded depends on how long the mixture sits in the sunlight. Once the reaction is complete, we can remove the MOF by filtering it out of the water. Then, we can collect the hydrogen and clean water!
How It Works
When sunlight excites the MOF, a kind of relay race begins! The relay batons (electrons) pass between ligands and metals within the framework. The electrons pass between many ligands and metals until they reach the surface of the MOF crystal. Most hydrogen-producing reactions occur at the surface. From the surface of the MOF, the electron is passed to a neighboring water molecule. The electrons from the MOF split the water molecule to produce hydrogen (H2) during the reaction. But the MOF cannot have an infinite number of electrons, right? What if it runs out? Before running out of electrons, the MOF steals them from neighboring molecules in the dirty water. The MOF can purify water by stealing the electrons from the pollutants and thus destroying the pollutants. Splitting water and breaking down pollutants occurs millions of times each second when the MOF is exposed to light! This reaction is summarized in Figure 3.
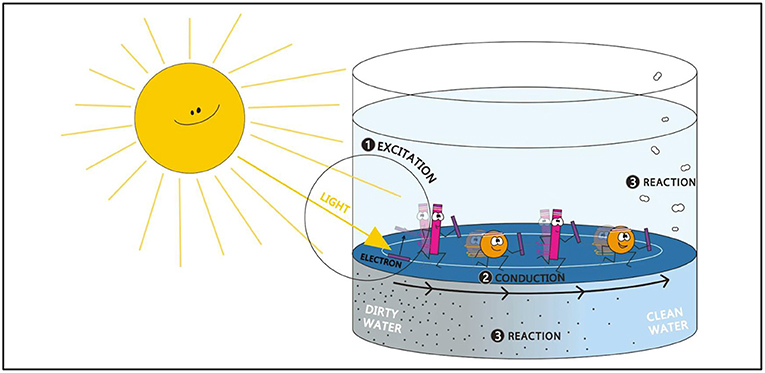
- Figure 3 - The MOF material is added to dirty water.
- When sunlight hits the MOF material, it provides energy for the reaction (relay race) to begin (step 1). The MOF passes the relay batons (electrons) from pollutants and gives them to water (step 2). When water gets the electrons, it is split apart to generate hydrogen fuel, and the pollutants are destroyed in the process (step 3).
Back to the Bigger Picture
We rely on the Earth and its natural resources for every aspect of our lives. Sadly, our reliance on fossil fuels has caused greenhouse gas pollution. A large amount of greenhouse gas in our atmosphere is causing climate change. We have reached the point where alternatives for fossil fuels are crucial for reversing the effects of climate change. MOFs are photocatalysts that can use sunlight to break down pollutants in water and produce hydrogen. MOFs can make hydrogen—an environmentally friendly and sustainable fuel—while cleaning water! It is exciting to see sustainable and innovative solutions to help solve two problems at the same time!
Glossary
Fossil Fuels: ↑ Fuels (e.g., oil, coal, natural gas) that come from ancient plants and animals that lived millions of years ago which are burned for energy.
Greenhouse Gases: ↑ Gases produced by burning fossil fuels that build up in the atmosphere and trap heat at Earth’s surface.
Climate Change: ↑ Burning fossil fuels releases greenhouse gases, which trap heat near Earth and cause our atmosphere to heat up quickly, making extreme weather events (e.g., forest fires, heatwaves, flooding) more common.
Catalyst: ↑ A material that assists or speeds up a chemical reaction.
Photocatalyst: ↑ A material that assists or speeds up a chemical reaction with the help of light.
Metal-Organic Framework (MOF): ↑ A porous, crystalline material made from metal atoms and organic linking molecules.
Ligand: ↑ Organic molecules (contain carbon atoms and are non-metals) that connect metal atoms to form one, two or three-dimensional metal-organic frameworks.
Electrons: ↑ Negatively charged atomic particles that can participate in chemical reactions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
KCS thanks the Department of Chemistry at Oregon State University for support through SciRis-ii and start-up funding, and Drs. S. Kampouri and T. N. Nguyen for useful discussions on the investigation of MOFs for water splitting. MTN and TR acknowledge support from the Department of Chemistry for the Milton Harris Graduate and Benedict Graduate fellowships, respectively. The authors thank Annie R. Thomas from the College of Science at Oregon State University for their help with figure illustrations.
References
[1] ↑ Tyra, B. 2021. Net Generation by Energy Source. Electric Power Monthly. Washington, DC: U.S. Energy Information Administration.
[2] ↑ Halmann, M. M., and Steinberg, M. 1999. Greenhouse Gas Carbon Dioxide Mitigation. Boca Raton, FL: CRC Press LLC.
[3] ↑ IPCC. 2018. “Summary for policymakers,” in Global Warming of 1.5°C, eds V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani (Geneva: World Meteorological Organization). p. 32.
[4] ↑ World Health Organization. 2021. Progress on Household Drinking Water, Sanitation, and Hygiene 2000-2020: Five Years Into the SDGs. Geneva: World Health Organization (WHO); the United Nations Children’s Fund (UNICEF).
[5] ↑ Kampouri, S., Nguyen, T. N., Spodaryk, M., Palgrave, R. G., Zuttel, A., Smit, B., et al. 2018. Concurrent photocatalytic hydrogen generation and dye degradation using MIL-125-NH2 under visible light irradiation. Adv. Funct. Mater. 28:1806368. doi: 10.1002/adfm.201806368