Abstract
Climate change is occurring today because of a buildup of carbon dioxide (CO2) in the atmosphere. This buildup of CO2 is mostly from burning fossil fuels for our energy needs. The oceans take up and store a lot of CO2 from the atmosphere. To know how much CO2 the oceans take up, we must understand the processes involved. These processes include the mixing of ocean water. Turbulent mixing is a fast and effective way to mix up ocean water, and it happens when for example wind blows over the ocean and creates waves. However, it is difficult to measure turbulent mixing close to the ocean surface. In this article, we describe how we overcame this problem and how we used our measurements to learn about the exchange of carbon dioxide between the atmosphere and the oceans.
The Role of the Oceans in a Changing Climate
Rapid climate change is a major environmental problem of our times. This climate change is human-made: release of greenhouse gases from cars, power plants, and other man-made sources increases the concentration of carbon dioxide (CO2)—one of the main greenhouse gases—in the atmosphere. The CO2 concentration in the atmosphere has currently reached its highest level in more than 600,000 years. Greenhouse gases in the atmosphere trap some of the energy that would otherwise escape into space, and they reflect that energy back to the Earth. This process increases the overall temperature at the Earth’s surface. The greenhouse effect is actually a good thing because it keeps the Earth’s average temperature at +15 °C instead of -18°C. The problem is that, currently, the concentration of greenhouse gases in the atmosphere is too high, and this has an enormous impact on our climate.
The oceans also play a role in climate change. An increase in CO2 has also been observed in the oceans. This is a direct effect of the rising atmospheric concentrations of CO2. The oceans absorb CO2 from the atmosphere in an attempt to balance out CO2 concentrations (nature loves balance). Think of a teabag in a bowl of hot water (Figure 1). The tea spreads from the high concentration in the bag to a lower concentration in the bowl. This process is called diffusion. In our real-world example, the atmosphere contains more CO2 than the oceans do. Thus, the CO2 diffuses into the oceans. The oceans absorb CO2 as long as the concentration of CO2 in the atmosphere is higher than in it is in the oceans. When we burn more fossil fuels, more CO2 is released into the atmosphere, and thus the oceans absorb more CO2. The oceans cover two-thirds of the Earth’s surface, so they can accumulate and store a large amount of atmospheric CO2. We are very interested in understanding how the oceans absorb CO2. This knowledge can help scientists to predict Earth’s future climate.

- Figure 1 - When a teabag is placed into a bowl of hot water, the tea diffuses from an area of high concentration (the bag) into an area of lower concentration (the water).
Ocean Turbulence and How to Measure It
The diffusion of gases between the atmosphere and the oceans is very slow. The tea from the teabag needs some time to spread through our bowl of water. However, you might have experienced that you can speed up the process by stirring the water with a spoon. Stirring creates turbulence in the water. Turbulence is an irregular motion of the water, and it causes faster and more efficient in spreading of the tea than does diffusion alone. The same happens at the interface between the atmosphere and the oceans. The CO2 uptake through diffusion is very slow, but when the ocean water is turbulent, this process is much faster. This means that the amount of turbulence determines the speed at which gases are exchanged between the atmosphere and the oceans. When there are many breaking waves and whitecaps, the gases exchange faster than when the oceans are calm (Figure 2).

- Figure 2 - Turbulent and calm sea conditions create different rates of gas exchange between the atmosphere and the oceans.
How do we know how much CO2 goes into the oceans? Through careful analysis! We need to understand the processes involved in gas exchange between the oceans and the atmosphere so that we can help to solve our planet’s climate crisis. Measuring ocean turbulence is difficult, especially close to the ocean surface. There are several instruments used to measure turbulence. Typically, scientists go out on ships and set the instruments into the ocean water. The scientists do not want to lose their instruments or the data that the instruments collect, so they tie the instruments to the ship with ropes and cables. However, this causes a major problem: when the instruments measure turbulence close to the ocean’s surface, they are actually measuring the turbulence created by the ship’s propeller. This is not the natural turbulence that we are interested in! Due to this problem, existing theories about how ocean turbulence drives gas exchange cannot be proven. Such theories were previously tested only in laboratories, lakes, and coastal regions. In these water bodies, it is easier to perform turbulence measurements than it is in the open oceans.
How Can We Solve the Problem of Measuring Turbulence?
To overcome the problem caused by a ship’s propeller, there are several approaches for measuring turbulence close to the ocean surface. These approaches include buoys that drift freely on the ocean surface [1] or methods that involve a special type of images of the ocean surface [2]. Another way to measure turbulence close to the surface is called the air-sea interaction profiler (ASIP) [3]. ASIP was developed by Brian Ward (an author of this article) to focus on what is happening at the air-sea interface. ASIP can be set into the ocean without being connected to the ship—we just put it into the ocean and then move the ship out of the area (Figure 3). Once it is in the water, ASIP is pulled from the surface down to a pre-defined depth (a maximum of 100 meters) by a set of thrusters on its lower end (Figure 3A). The thrusters turn off once the intended depth is reached. ASIP will then rise right back to the ocean surface under its own lifting force, like when you press a floating object under water and then it rises again. As it rises, ASIP measures the water shear, which is created when the water particles move with different speed. We can use water shear to estimate the level of turbulence. ASIP is designed to measure as it rises, so that the turbulence created by the thrusters does not disturb the measurement. The thrusters and sensors on ASIP are powered by an internal battery. Since the thrusters use the most energy, the number of dives and their depths determine how long ASIP can conduct measurements. The battery lifetime allows for roughly 6,000 meters of total measuring, for example, ~60 measurements from 100 meters deep. Of course, we do not want to lose the profiler in the ocean, so it is equipped with a global positioning system (GPS) that sends its exact position to a satellite (like the navigation apps on mobile phones) each time ASIP reaches the ocean’s surface. We receive the position from the satellite, so we can pick up ASIP whenever we want. All the data are stored inside ASIP, and we can download that data when ASIP is recovered.
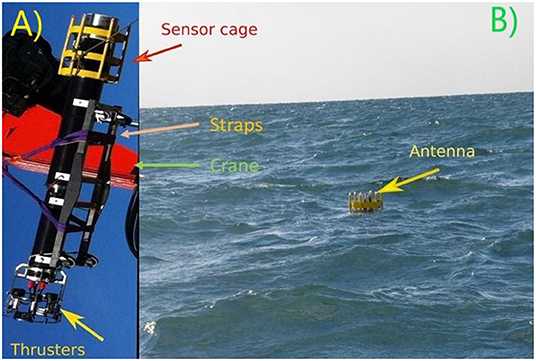
- Figure 3 - The air-sea interaction profiler (ASIP).
- (A) ASIP hanging on a crane, with its thrusters visible. (B) ASIP in the ocean, with only the sensor cage above the water.
Our Findings in the Open Ocean
For our study, we used ASIP in the Atlantic Ocean, from a research vessel called Knorr. We started in Woods Hole (USA) in late June of 2012 and returned in the middle of July. During the cruise we measured ocean turbulence with ASIP. We also measured the levels of atmospheric and oceanic CO2 using instruments installed on the ship. From our measurements we showed, for the first time in the open ocean, that ocean turbulence speeds up air-sea gas exchange.
Using ASIP’s measurements, we showed that the turbulence near the ocean surface could explain CO2 exchange well. The data also allowed us to improve existing theories. For example, we could use the data to improve the description of surface roughness, which tells us how flat or wavy the ocean surface is. Scientists used to believe that the ocean surface was always completely wavy, but sometimes it is rather flat, like when there is no wind or when there is an oily film on the surface.
Our results are important because they provide an accurate description of the CO2 exchange between the atmosphere and the oceans. These data can help scientists to predict Earth’s future climate as accurately as possible, which could help people to prepare for the effects that for example warmer temperatures cause. Such effects can be droughts or extreme heats in summer. Also, the predictions can help to convince people to consume less CO2 and avoid an even further increase of the global temperature.
Glossary
Climate Change: ↑ A change in the patterns of the weather conditions over a long time, for example an increase in the global temperature over a long time period.
Greenhouse Gases: ↑ Gases in the atmosphere that absorb and emit heat energy, increasing the temperature at the Earth’s surface.
Carbon Dioxide (CO2): ↑ A gas that occurs naturally in Earth’s atmosphere. It is also emitted by burning of fossil fuels. It is the most significant greenhouse gas in Earth’s atmosphere.
Diffusion: ↑ The movement of particles from an area of higher concentration to an area of lower concentration, continuing until a balance is reached.
Turbulence: ↑ An irregular motion of air or water that is characterized by up-and-down currents.
Air-Sea Interaction Profiler (ASIP): ↑ Instrument to measure among others turbulence close in the uppermost 100 meters of the ocean.
Water Shear: ↑ Shear is created when water particles that touch each other move with different speed.
Global Positioning System (GPS): ↑ A system that uses signals from satellites to determine a location on the Earth’s surface.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Original Source Article
↑Esters, L., Landwehr, S., Sutherland, G., Bell, T. G., Christensen, K. H., Saltzman, E. S., et al. 2017. Parameterizing air-sea gas transfer velocity with dissipation. J. Geophys. Res. Oceans 122:3041–56. doi: 10.1002/2016JC012088
References
[1] ↑ Thompson, J. 2012. Wave breaking dissipation observed with “SWIFT” drifters. J. Atmos. Oceanic Technol. 29:1866–82. doi: 10.1175/JTECH-D-12-00018.1
[2] ↑ Veron, F., Kendall Melville, W., and Lenain, L. 2009. ASIP: measurements of ocean surface turbulence and wave–turbulence interactions. J. Phys. Oceanogr. 39:2310–23. doi: 10.1175/2009JPO4019.1
[3] ↑ ten Doeschate, A., Sutherland, G., Esters, L., Wain, D., Walesby, K., and Ward, B. 2017. ASIP: profiling the upper ocean. Oceanography 30:33–5. doi: 10.5670/oceanog.2017.216