Abstract
Our cells have their own recycling center! Autophagy is the name of this amazing process, which allows cells to recycle cellular components to generate new ones and to provide energy for cell survival. If we eat too much high-fat food, autophagy might start malfunctioning in specific parts of the brain, and this can contribute to the development of obesity. Therefore, eating healthy food is important, so we can keep autophagy working properly and prevent obesity!
Autophagy: The Waste-Recycling System of Our Cells
Within our cells, many processes occur simultaneously. Various cellular components work together to maintain the cells and keep the body healthy. However, these cellular components can get old and in need of replacing, or they can accidentally get damaged. When this happens, the “waste alert” within the cell turns on, and a process called autophagy comes into action. Autophagy is a process that occurs in the cytoplasm of cells. The mission of autophagy is to collect cellular waste and to deliver it to the cell’s recycling center, where the waste can be used to generate energy or to make new cellular components from old ones. Without autophagy, cells would die from the negative effects of waste accumulation.
Autophagy consists of several steps. The structures that form in these steps may have complicated names, but you can easily follow the process in Figure 1. First, the cell’s endoplasmic reticulum donates a piece of its membrane to form a structure called a phagophore. The phagophore begins to enclose the cellular waste, including old or damaged cell parts and proteins. The phagophore then totally closes up around the waste, forming a spherical structure called an autophagosome. You can imagine this process as a soap bubble forming around the waste material that needs to be recycled. The autophagosome travels to the recycling factory of the cell: the lysosome. The fusion of the lysosome with the autophagosome forms the autolysosome, where the magic happens. Specialized proteins from the lysosome break down the cellular waste to generate new material that will be used to build new cellular components and to create energy.
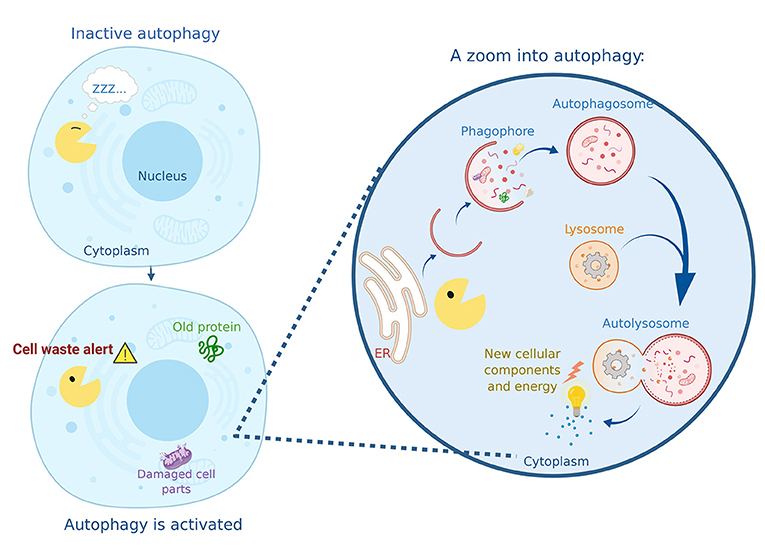
- Figure 1 - Autophagy in action.
- “Autophagy Man” (yellow) wakes up when he has a mission: to collect cell waste and deliver it to the recycling center. Autophagy Man begins to capture damaged cell parts and aging proteins into his phagophore, then he wraps them in a double membrane called an autophagosome. Once filled with the material that must be removed, the autophagosome reaches the lysosome and fuses with it, generating a new structure called the autolysosome. This is where the degradation of the cargo occurs, resulting in the generation of energy and the production of new cellular components.
Autophagy Saves Our Cells When There Is No Food!
Since our cells are always working and wearing out, autophagy is always occurring. However, autophagy may work more than usual in certain situations, for example when cells are in conditions of starvation or nutrient deprivation. Our bodies and cells normally receive energy through the foods we eat. The nutrients from the carbohydrates, fats, and proteins that we ingest are either transformed directly into the energy needed for bodily functions and physical activity or, if they are not immediately needed, the nutrients are stored in our cells until the body requires them.
When we skip meals, for example during a period of fasting, we are not taking energy and nutrients into our bodies. A low-energy alert turns on within our cells and stimulates autophagy, which supplies the energy that is not being provided by food. Autophagy accomplishes this by breaking down the cell’s nutrient stores, which are then used to obtain energy. In this emergency situation, autophagy is still recycling cell waste, but it will prioritize the breakdown of nutrient stores to meet the energy requirements of our cells. In this way, autophagy maintains energy balance in the body and prevents cells from dying of starvation (Figure 2) [1].
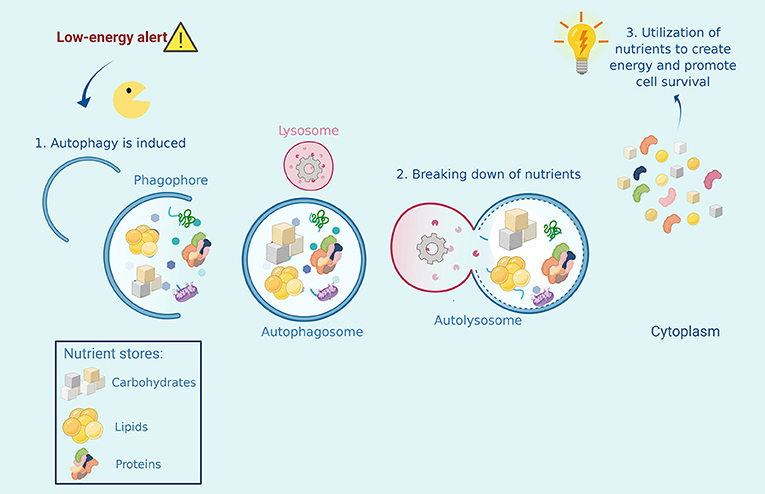
- Figure 2 - Autophagy helps cells that are experiencing starvation or nutrient deprivation.
- When a low energy alert is activated in the cell, Autophagy Man must collect stores of nutrients inside the cell, such as carbohydrates, proteins, and lipids, and bring them to the recycling center, where he fuses with a lysosome. The broken-down nutrients are then converted into energy that the cell can use to survive.
Overeating Stops Autophagy in the Brain
Now we know that autophagy senses the decrease in incoming nutrients that indicates starvation. Importantly, autophagy also senses overeating! What happens when we eat too much? If we eat more than our bodies need, autophagy can malfunction and this may contribute to the onset of obesity. This is worrying because obesity is currently an epidemic in some countries. The latest report from the World Health Organization indicates that, in 2016, 39% of adults worldwide were overweight, while 13% were obese. And rates are only getting higher each year!
To understand the relationship between autophagy and obesity, we must first know that one of the main contributors to increasing obesity rates is the overconsumption of foods rich in saturated fats. An increased concentration of saturated fats in our bodies can cause several different diseases. Saturated fats from high-fat diets can accumulate in the brain and affect its function [2–4]. Let us focus our attention on the hypothalamus, a region of the brain involved in the regulation of appetite. When saturated fats accumulate in the cells of the hypothalamus, the normal regulation of appetite is lost.
How does this happen? In the hypothalamus, a group of specialized brain cells normally knows when we are hungry or full because they receive information from other parts of the body through chemical messengers, like insulin. When we eat, insulin is released from the pancreas and travels through the blood to the brain, informing cells in the hypothalamus that the body has enough food. In response to insulin, the hypothalamus produces a satiety (“I am full!”) signal that causes us to stop eating.
Since it is complicated to study the brain in humans, scientists (including us!) have studied mice to understand how saturated fats affect autophagy in the brain, thereby altering the regulation of appetite, for more information see “From Mice to Humans: The Study of Obesity” [5]. We have seen that, when mice chronically consume high-fat diets (for 16 weeks), saturated fats accumulate in their brains [6]. Autophagy takes place to try to keep the fat in check. Imagine autophagy in a battle against fat accumulation, fighting to break down the excess fat stores. Unfortunately, if there is too much fat, autophagy will lose. Once this happens, autophagy is inhibited, which means that it will no longer be able to fight against fat accumulation. Fat accumulation will eventually cause damage to brain cells, preventing their ability to sense insulin, and the hypothalamus will no longer give the satiety signal [7]. As a consequence, more and more food is consumed, eventually leading to obesity (Figure 3).
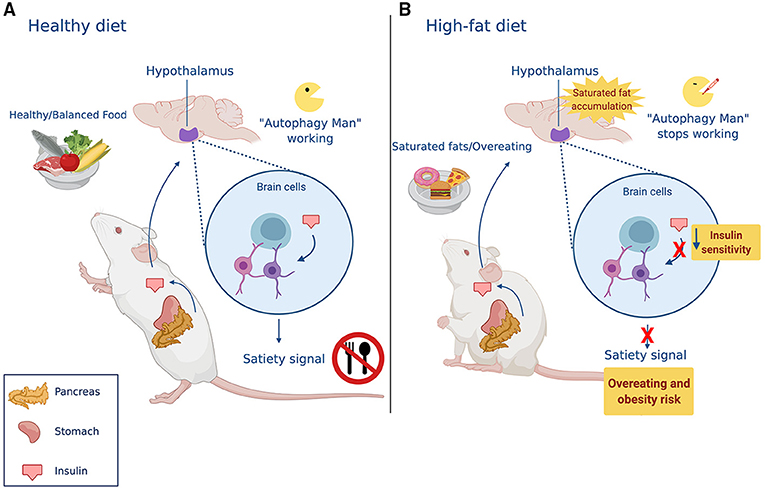
- Figure 3 - Autophagy regulates how much animals eat.
- (A) In healthy conditions, eating stimulates the release of insulin from the pancreas. Once the hypothalamus receives the signal transmitted by insulin, it understands that the body has received enough food, and specialized brain cells send a “stop eating” signal. (B) A high-fat diet causes the accumulation of saturated fats in the brain. This built-up fat inhibits Autophagy Man’s super-powers, and brain cells lose the ability to recognize insulin’s message. This causes the mice to eat more than they need.
There are many different types of saturated fats and we do not know if all of them can turn off autophagy in the hypothalamus. In our lab, we tested palmitic acid, which is the most common saturated fat found in most high-fat diets. Palmitic acid can be found in foods like red meat, dairy, butter, and palm oil. Using cell cultures of hypothalamic cells, our experiments showed that palmitic acid inhibits autophagy and reduces the cells’ ability to detect insulin [8].
What About the Future?
Autophagy is an essential process in all cells. While its primary function is breaking down cellular waste, it also regulates the cellular response to nutrients. In summary, the consumption of a high-fat diet causes an accumulation of saturated fats in the brains of mice, inhibiting autophagy in the cells of the hypothalamus. When autophagy stops, these brain cells can no longer respond to insulin nor give the satiety signal. We hypothesize that the same is happening in humans [5], however future research is needed to confirm that this is the case.
The excessive consumption of saturated fats is detrimental to our health. Today’s treatments for obesity do not consider the role that saturated fats, such as palmitic acid, might be playing. Furthermore, obesity treatments do not consider the role of autophagy. Failure to take these important processes into account could explain the limited effectiveness of many obesity treatments. Drugs that can manage obesity by regulating autophagy have been identified in animals and there are ongoing tests to determine whether these drugs also work in humans. Once we fully understand how saturated fats affect autophagy in the brain cells that regulate appetite, we may be able to develop new therapies that help autophagy to work better when fat accumulates in the brain. Such treatments would save many lives and would help to slow the world’s worsening obesity epidemic.
Glossary
Autophagy: ↑ The cellular process that collect unnecessary or old cellular components either to removed them, through a process of degradation, or to recycle them to build new cellular components.
Saturated Fats: ↑ An unhealthy type of dietary fat that can cause several diseases if eaten in excess.
Hypothalamus: ↑ Hypothalamus is a brain region that regulates sleep cycles (when to sleep), body temperature, food intake (when to eat and spot eating), and other nervous system functions.
Insulin: ↑ A hormone (signaling molecule) that is released into the bloodstream from the pancreas after eating, causing the hypothalamus to send an “I am full” message.
Satiety: ↑ The feeling of being full, which indicates that the body has no immediate need to eat.
Palmitic Acid: ↑ Palmitic acid is the most common saturated fat, we find it in foods such as meats, cheeses, vegetable oils and others.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was founded by grants from Fondo Nacional de Desarrollo Científico y Tecnológico, FONDECYT 1200499 to EM and by the CONICYT PIA 172066 to EM. Figures have been realized using the program BioRender. The authors thank everyone in the Morselli lab for constructive discussions and criticisms.
References
[1] ↑ Kim, K. H., and Lee, M. S. 2014. Autophagy—a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 10:322. doi: 10.1038/nrendo.2014.35
[2] ↑ Karmi, A., Iozzo, P., Viljanen, A., Hirvonen, J., Fielding, B. A., Virtanen, K., et al. 2010. Increased brain fatty acid uptake in metabolic syndrome. Diabetes 59:2171–7. doi: 10.2337/db09-0138
[3] ↑ Valdearcos, M., Robblee, M. M., Benjamin, D. I., Nomura, D. K., Xu, A. W., and Koliwad, S. K. 2014. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 9:2124–38. doi: 10.1016/j.celrep.2014.11.018
[4] ↑ Morselli, E., Criollo, A., Rodriguez-Navas, C., and Clegg, D. J. 2014. Chronic high fat diet consumption impairs metabolic health of male mice. Inflamm Cell Signal. 1:e561.
[5] ↑ Weiskirchen, S., Weiskirchen, R., and Weiper, K. 2020. From mice to humans: the study of obesity. Front. Young Minds. 8:92. doi: 10.3389/frym.2020.00092
[6] ↑ Rodriguez-Navas, C., Morselli, E., and Clegg, D. J. 2016. Sexually dimorphic brain fatty acid composition in low and high fat diet-fed mice. Mol. Metab. 5:680–9. doi: 10.1016/j.molmet.2016.06.014
[7] ↑ Meng, Q., and Cai, D. 2011. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase β (IKKβ)/NF-κB pathway. J. Biol. Chem. 286:32324–32. doi: 10.1074/jbc.M111.254417
[8] ↑ Hernández-Cáceres, M. P., Toledo-Valenzuela, L., Díaz-Castro, F., Ávalos, Y., Burgos, P., Narro, C., et al. 2019. Palmitic acid reduces the autophagic flux and insulin sensitivity through the activation of the free fatty acid receptor 1 (FFAR1) in the hypothalamic neuronal cell line N43/5. Front. Endocrinol. 10:176. doi: 10.3389/fendo.2019.00176