Abstract
The ocean is continuously in motion. A large part of this motion is driven by water with different temperatures and different concentrations of dissolved salts in different areas of the ocean. In this article, we will discuss how these differences in ocean water can create ocean currents. We also tell you how to perform a simple experiment that can easily be done at home, to illustrate the formation of ocean currents.
Forever in Motion: The Global Conveyor Belt
Although we are often taught to think of oceans like the Atlantic Ocean or the Pacific Ocean as separate from one another, in reality all ocean basins are connected together, forming one enormous ocean [1]. In this enormous ocean, which extends all around the world, water moves out of one basin and into another. On its journey around the globe, the water transports heat and salt from the tropics to the earth's poles, nutrients from the ocean's depths to the surface, and fresh water entering at the coast (from rivers or melting glaciers) out into the sea.
Even though the ocean is constantly in motion and there are lots of factors that affect how the ocean water moves, there is one natural phenomenon that has been contributing to ocean water movement for thousands of years: one large ocean current that connects all ocean basins, as well as the ocean surface and the deep ocean. This current is sometimes called the global conveyor belt, for the way it circulates water all around the globe (Figure 1). If water could be tracked on its journey on the global conveyor belt, following the red path as warm water nears the surface, then cools and sinks to follow the blue path until it comes back to the surface again, we would find that it takes the water around 1,000 years to complete its travel all the way around the world.
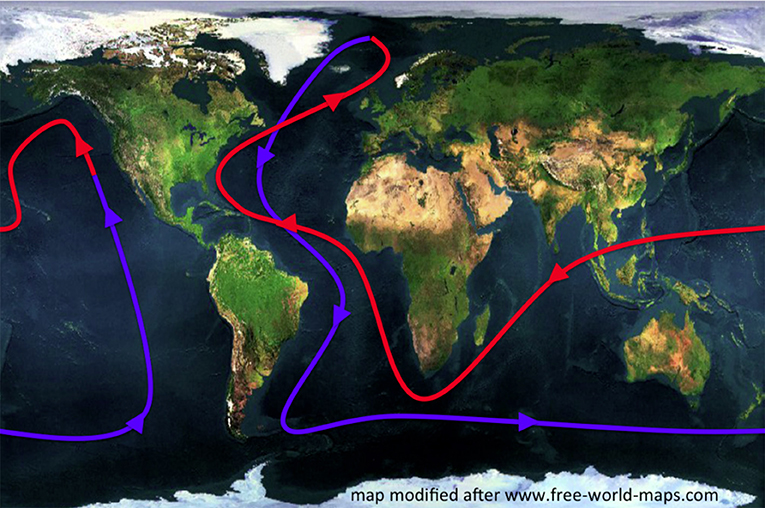
- Figure 1 - The global conveyor belt.
- Warm ocean currents near the surface are shown in red, cold ocean currents near the bottom are drawn in blue. Due to these currents, water travels, over the course of about 1,000 years, all around the globe. Sketch after Rahmstorf [2] on top of a map from http://www.free-world-maps.com.
Gone With The Wind: Winds Drive Ocean Circulation
An obvious first guess as to what might cause this motion in the ocean is the wind. Wind blows over the ocean's surface, causing both waves and movement of water in a downwind direction. And indeed, parts of the global conveyor belt are driven by the wind [3]. Wind systems, like the trade winds for example, consist of strong winds that constantly act on large surface areas of the ocean, supplying large amounts of energy and putting huge volumes of water into motion.
Density-Driven Ocean Circulation
However, another part of the motion of ocean water in the global conveyor belt is caused by something much less obvious: density differences in the water. Density is a measure how heavy a certain amount of a substance is. The definition of density is thus mass per unit volume. For example, a cube of whipped cream has a much lower density, and thus a lower mass, than a cube of rock of the same size Box 1.
BOX 1 - WHY DO WE NEED TO LEARN ABOUT DENSITY ANYWAY?
Apart from its importance in understanding ocean circulation, why would you want to know a substance's density? There are several reasons. Sometimes it is easier to measure a volume than the weight of something. For example when baking, you have probably come across those graduated measuring cups with different scales down the side, indicating how far you would have to fill the cup for a certain weight of sugar, flour, water, and others. Why is it not enough to have just one scale on the cup for all of the ingredients? Because 1 cup of water takes less space than 1 cup of flour. This is means that 1 cup of water is more compact, has a higher density, than 1 cup of flour.
Imagine an ice cube tray filled with water all the way up to the rim. When you place this ice cube tray into the freezer and come back the next day, you will find that the ice cubes have grown and now bulge out of the tray. The water that you put into the ice cube tray now takes up a larger volume than it did before it was frozen. So, if you wanted the ice cube tray filled with ice exactly up to the rim, the way you initially filled it, you would have to shave away the bulge in the ice, which would decrease the mass of water left in the tray. This tells us that ice has a lower density (is less compact) than liquid water, because the same mass of water spreads out and takes up more space when it is frozen. Therefore, when you place ice cubes in water, they will float at the surface.
A similar thing happens for two liquids: If liquids have different densities, the denser liquid sinks to the bottom and the lighter one floats on top. For example, if you pour oil on water, the oil will float on top of the water. If you pour water on oil, the water will sink through the oil and spread underneath it, pushing the oil up to the surface. This happens in the ocean, too: If, for some reason, water near the surface of the ocean becomes denser than the water below, the denser water will sink down, displacing the less dense water, which will rise to the surface.
What Causes Density Differences?
In the ocean, density is determined by several factors, including the amount of pressure the water is under, how much salt is dissolved in the water, and the water's temperature. The more pressure water is under, the more it is compressed, and thus the higher its density becomes. The pressure in the ocean increases a lot as you dive downwards. The ocean is, on average, 4 km deep, and at those depths, pressures are very high. Density is also affected by how much salt is dissolved in the water. The salt content of sea water is called its salinity, and the higher the water's salinity, the higher its density. The typical salinity of ocean water is 35 grams per liter, which is equivalent to ~7 teaspoons of table salt per 1 liter of water (or 2 teaspoons per cup of water). Last, the water's temperature influences its density. In general, the colder the water, the closer the molecules squeeze together, meaning the less space they take up and the higher the density.
Since temperature, salinity and pressure are different at different places throughout the world's ocean, the seawater's density is different in different places, too. In Figure 1, we saw the ocean currents of the global conveyor belt spanning the whole globe. In the far north, the warm (red) surface current cools and sinks, turning into a cold (blue) current deep in the ocean. This is because the colder water has a higher density than the warmer water.
Kitchen Oceanography: Ice Melting in Freshwater and Saltwater
Now that we have seen that density differences in the ocean are helping to drive ocean currents, let us do a simple experiment that will help this idea to become much more clear.
Question: If you take two ice cubes of similar size and place them into room-temperature water, one in freshwater and one in saltwater, which ice cube will melt faster?
Hypothesis: How quickly the ice cubes melt depends on the temperature of the water that surrounds them. Melt water from the ice cubes is colder than the room-temperature water the ice cubes are placed in, therefore ice cubes surrounded by their own melt water will melt more slowly.
Prediction: The ice cube in freshwater will melt faster, because the cold melt water from the ice cube is denser than the freshwater and will thus sink down and away from the ice cube. The ice cube placed in saltwater, on the other hand, will be surrounded by its own, cold melt water, because the freshwater will float on the saltwater, which is denser. Thus, the ice cube in saltwater will melt more slowly.
Experiment: Put one ice cube into room-temperature freshwater and another into room-temperature saltwater and observe! For saltwater, you can use a salt concentration similar to that of typical ocean water (see above). To make it easier to observe the melting of the ice cubes and where the water goes, it might be helpful to add food coloring to the water before freezing the ice cubes.
Results: In Figure 2, the results of this experiment are shown and described. This experiment helps us distinguish three different “water masses,” with three different densities: (1) room-temperature saltwater, which is the densest of the three types of water; (2) cold and fresh melt water from ice cubes, which is less dense than the saltwater, and thus floats on top of it; and (3) room-temperature freshwater, the least dense of the three, through which the cold and fresh melt water sinks.
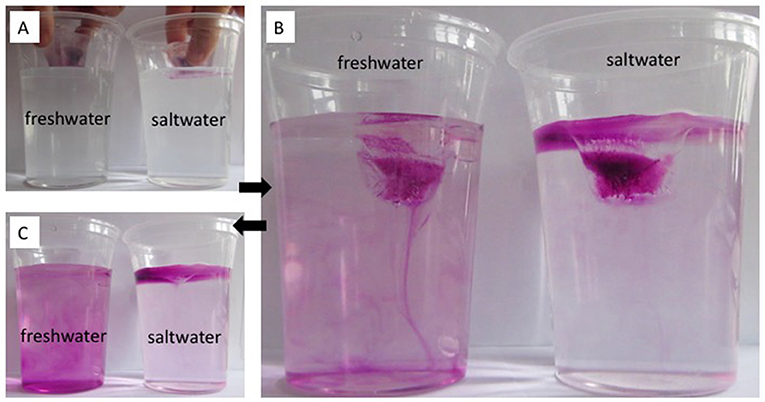
- Figure 2 - (A) Colored ice cubes are placed into room-temperature freshwater and saltwater. Melting is observed over time.
- (B) In the freshwater beaker, the colored melt water sinks down. In the saltwater beaker, it stays at the surface and spreads there. (C) By the end of the experiment, the beaker previously containing freshwater has mixed with the melt water, while in the salt water beaker the melt water still floats at the surface. (Photos: Mirjam S. Glessmer).
What are the Consequences of Changing Water Densities in The World's Ocean?
The experiment with ice cubes demonstrates how different densities of water influence water circulation: less dense water will spread on denser water, denser water will sink through less dense water and spread below it. This is exactly what happens in the ocean! But, let us also consider a different scenario: If freshwater is introduced into the ocean in regions where cold ocean water is sinking down to form the deep branch of the global conveyor belt, for example by glaciers melting, this fresh water will spread on top of the ocean and not sink, insulating the deeper ocean from the cold atmosphere above, especially if the fresh water freezes. This will have some effect on how the ocean's circulation patterns develop over the next years and decades, in combination with other factors, like the winds. This is an exciting area of active research!
Notes:
- A movie of this experiment can be seen here: https://mirjamglessmer.com/2013/09/01/ice-cubes-melting-in-salt-water-and-freshwater-post-13/
- For more kitchen oceanography experiments, see mirjamglessmer.com/kitchen-oceanography
Glossary
Density: ↑ Density is a measure how heavy a certain amount of a substance is.
Ocean Circulation: ↑ The movement of water with ocean currents, e.g., the Gulf Stream.
Salinity: ↑ The salt content in sea water; how “salty” the sea water is.
Circulation Pattern: ↑ Persistent locations of ocean currents.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Ocean Literacy: The Essential Principles of Ocean Sciences for Learners of All Ages, Version 2: March 2013. Available online at: http://www.coexploration.org/oceanliteracy/documents/OceanLitChart.pdf
[2] ↑ Rahmstorf, S. 2006. “Thermohaline ocean circulation,“ in Encyclopedia of Quaternary Sciences, ed S. A. Elias (Amsterdam: Elsevier).
[3] ↑ Bringedal, C., Eldevik, T., Skagseth, Ø., Spall, M. A., and Østerhus, S. 2018. Structure and forcing of observed exchanges across the Greenland–Scotland Ridge. J. Clim. 31:9881–901. doi: 10.1175/JCLI-D-17-0889.1

