Abstract
We see ice all the time: as cubes in drinks, forming on pools of rainwater in winter, in movies like Frozen or The Blue Planet. But have you ever wondered how exactly ice forms and what determines what it looks like in the end? In this article, we investigate how the formation of ice is influenced by the movement of water while it is freezing, by the direction the cooling is coming from, and by salt dissolved in the water.
Just Chilling: Ice Cubes in a Freezer
If you have a freezer in your kitchen, you can easily investigate ice formation for yourself. Get an ice cube tray, fill it with water, put it in the freezer, and come back the next day. If nobody opened the freezer after you put the tray of water inside, the ice cubes will have frozen slowly, to look like clear glass. This means the water molecules froze in an organized crystal structure.
But what if someone opened the freezer? If ice is disturbed while growing, it forms irregularities. This means that it will not look as clear, because not all of the molecules were well-organized as they froze. There might be layers going through the ice that are not transparent, and the surface might be less smooth.
Keeping it Moving: Waves Disturb Sea Ice Formation
In the sea, there are almost always disturbances occurring during ice formation, because there are almost always waves. When ice forms in the sea, it therefore looks different from ice formed in your freezer, and sea ice forms through several different stages.
First, there is a stage when the water is very cold, but still liquid (see the top left corner of Figure 1A). Then, there is a zone where ice has started to form in tiny needle-shaped crystals that come together to form ice slush (Figure 1A, in the middle). The slush slightly calms the waves coming in from the open water, but there are still enough waves to prevent the needles from freezing together and forming a smooth ice surface.

- Figure 1 - Stages of sea ice formation.
- (A) Open water (upper left), ice slush (middle), ice pancakes (lower right). (B) A thin layer of ice that was broken into larger ice floes after freezing. (C) Pancake ice. (D) Ice-covered ocean with a track created by a ship moving through. The surface of the sea ice is covered in snow (Photos: Mirjam S. Glessmer).
Sometimes, on a calm day when there are no waves disturbing the process, needles can freeze together and a layer of ice forms. Initially, this layer is transparent enough to see through and spot algae growing on the sandy bottom underneath (Figure 1B). After this thin layer forms, it can break up into several ice floes.
Usually, there are waves during ice formation, and you see pancake ice [Figures 1A (toward the bottom right), C]. Pancake ice forms when ice needles freeze together to form larger ice floes. As they are moved by waves, the ice floes bump into each other. The edges of the ice floes get rounded off by these collisions, and the bits that break off get pushed on top of the round ice floes, giving the ice pancakes rims around their edges. Over time, smaller pancakes freeze together to form larger and larger pancakes, and, eventually, they can freeze together to cover large areas of the ocean (Figure 1D).
Top-Down Business: Freezing a Lake or the Sea
But wait, why is there water underneath the ice? Why does the ice not form throughout the whole depth of the water, as it does with ice cubes in the freezer? Well, in a way, the ice formation in the sea is not finished yet. If it was cold enough for a long enough time, the whole ocean might freeze all the way through. But there are a couple of reasons why it is a lot more difficult for the ocean to freeze all the way through than for an ice cube to do so—and that is not just because there is more water in the ocean than in the freezer.
When ice forms in the freezer, the ice cube tray is surrounded by cold air on all sides. However, when water freezes in a lake or the ocean, the cold air is only above the water’s surface. Below and to the sides, the water is still surrounded by more water or the by sea/lake floor, which are warmer than the air. Therefore, cooling, and thus freezing, can only happen from top down.
Sometimes we can even see that ice forms from the top down. Figure 2 shows the frozen surface of a lake, with air bubbles inside the ice. How did those air bubbles get there? The bottom of the lake is muddy and sometimes air bubbles come out of the mud and bubble up through the water, all the way to the surface. Once there is a thin layer of ice covering the lake, the air bubbles cannot escape from the water and are trapped below the ice. As the cooling from the air above continues, the ice continues growing downward into the water and forms around the bubbles, until the ice is eventually thick enough to fully enclose the bubbles, one by one. As more air bubbles up, those bubbles are also trapped inside the ever-thickening ice underneath the first layer of bubbles.

- Figure 2 - A lake freezing from the top down, trapping air bubbles from the muddy lake bottom in the ice (Photos: Mirjam S. Glessmer).
Cold All Around: Ice Cubes in Your Freezer
If you have an old freezer, maybe even with the cooling element visible on the icy back wall, ice cubes might not freeze equally from all sides. To observe this, add food coloring to water before putting the ice cube tray in the freezer. Food coloring does not fit in the organized crystal structure that pure water forms when freezing. The color is excluded from the crystal structure, enriching the remaining liquid water with more color, making it more difficult to freeze. This can lead to interesting-looking ice cubes as shown in Figure 3. In Figure 3B, you can see that the ice cubes in the row toward the back of the freezer look very different from the ones in the row toward the front, even though they were all made from the same water, poured in the tray and put into the freezer at the same time. In the ice cubes in the bottom row of the tray pictured, ice has formed from all sides of the cube toward the center, excluding the color from its crystal structure and pushing it toward the middle. In Figure 3A and the top row of Figure 3B, you can see that, on one side, the clear layer of ice is thicker than on the other sides. That is the side that was facing the back wall with the cooling element, where ice formation was fastest. In ice cubes from the front row of the tray (Figure 3C), where cooling happened primarily from the top, the color froze out toward the bottom of the ice cube, leading to clear ice on top and colored ice below. This “cooling from above” is similar to the way a lake or the sea would freeze.

- Figure 3 - Ice formation experiments.
- (A) Ice cube that has frozen from all sides, thus pushing the color toward the middle. (B) The top row of ice cubes in the tray froze from one side toward the other, pushing the color to one side of the cubes, and the bottom row froze from the sides toward the middle, pushing the color toward the middle. (C) Ice cube that has frozen top to bottom, pushing the color toward the bottom. (D) Ice cubes frozen from fresh water (left) and salt water (right) with food coloring dripped on them to reveal their structures (Photos: Mirjam S. Glessmer).
Spicing It Up: Sea Water is Salty
What happened in this ice experiment with food coloring is very similar to what happens in the sea, and it also helps to explain why it is more difficult to freeze the sea than to freeze an ice cube made from tap water. Of course, seawater does not contain food coloring. But it does contain salt, which acts in a way that is similar to the food coloring. Unfortunately, salt in sea ice is not as clearly visible as food coloring, but there usually is a lot more salt in seawater than there was food coloring in the water used for the ice cubes in Figure 3. To prepare water with as much salt in it as typical seawater, 7 teaspoons full of salt must be added to one liter of tap water (a teaspoon holds about 5 grams of salt, sea water typically contains around 35 grams of salt per 1 liter of water). Freezing this salt water in an ice cube tray leads to several interesting observations. First, freezing takes longer than it does for freshwater ice cubes. Second, salt water ice cubes are not as transparent as freshwater ice cubes. And third, when the salt water ice cube is taken out of the ice cube tray, there is very likely some very salty, unfrozen water at the bottom of the tray.
To compare the structures of freshwater and salt water ice cubes, a small amount of food coloring can be carefully dripped onto the ice cubes (see Figure 3D). The ice frozen from salt water has a porous, almost sponge-like structure, through which the color seeps into the ice cube. Those are the pores into which the concentrated salt water was pushed while fresh water was forming ice all around. The fresh water ice cube, however, is a solid block of ice that the food coloring just runs off of.
So Cool: Observing Ice!
If we look at it closely, ice tells us so much about the way it formed. It is fascinating to look at ice and think about why it looks exactly the way it does. Was it interrupted during the freezing process? Which side did the cooling come from? Were substances dissolved in the water? Figure 4 shows a summary of what we have learned about ice formation. Next time you look at ice, you will be able to tell!
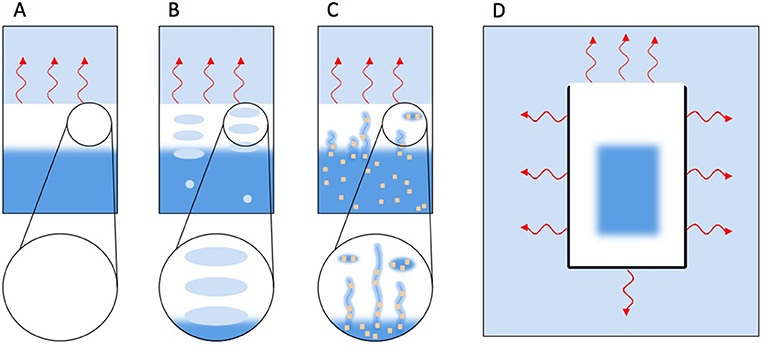
- Figure 4 - How different processes influence ice formation. Light blue indicates cold air, white ice, and dark blue (liquid) water.
- (A) Freshwater freezing top to bottom because of heat loss (red wriggly arrows) toward a colder atmosphere. (B) Freshwater freezing top to bottom but gradually enclosing air bubbles that rise from below. (C) Salt water freezing top to bottom, but pushing salt water into pores that stay as impurities in the ice. Salt is represented as yellow dots. (D) Ice cube freezing in an ice cube tray. Heat is lost to all sides, so ice is forming from all sides toward the middle.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.

