Abstract
For as long as anyone can remember, people have noticed that the brain has a limited ability to heal after injury. It was generally thought that this was in part due to the inability of the brain to make new cells. Then, researchers observed that there are two special regions of the brain that actually do produce new cells, even in adults. The cells from these two special regions are called neural stem cells and now scientists are working hard to determine how their special properties can be used to treat different types of damage in the brain.
What are Cells?
You have probably heard that humans are made up of mostly water. So, why don’t we collapse into puddles on the ground? That is because the human body is made up of all different types of cells – skin cells (which are really flat), heart cells (which beat – for real!), and brain cells (which transmit information), to name a few. Cells (Figure 1) have an important feature that prevents us from collapsing into puddles on the ground: they have an outer membrane, made up of special fat molecules, that keeps the water inside from leaking out! Inside the cells, there are more fatty membrane compartments called “organelles” that all have their own important jobs. One of the most important organelles is the nucleus. This is where the genetic information in the form of deoxyribonucleic acid (DNA) is found. The nucleus controls which different proteins are expressed in each different type of cell. The proteins are the busy workers in the cell because they perform important jobs to allow the cell to do what it needs to do!
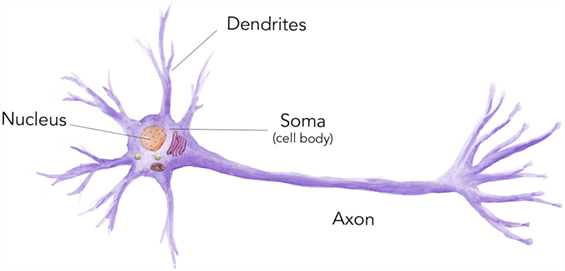
- Figure 1 - Neurons are one type of cell and they have a variety of organelles inside them, each with a very important function.
- For example, inside the nucleus, the instructions to make proteins are stored in the form of DNA.
What Types of “Specialized” Cells Do We have in the Brain?
There are several types of “specialized” cells in the brain such as neurons (Figure 1), oligodendrocytes, and astrocytes (Figure 2). We call these cells specialized because they have different shapes and properties that are designed to allow these cells to perform specific functions. Neurons, with their projections called “dendrites” and “axons,” enable the different regions of our brain to communicate with one another and allow the brain to talk to (and control) the rest of the body, which enables us to move about and sense changes in our environment. Neurons transmit and receive information. Oligodendrocytes wrap around the neurons, providing support that enables the neurons to transmit this information quickly. Astrocytes (star cells) support the nervous system by providing nutrition and regulating what can pass into the brain from the rest of the body.
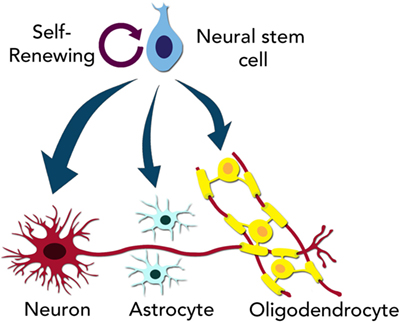
- Figure 2 - Neural stem cells are capable of “self-renewing.”
- This means they can give rise to another stem cell. However, neural stem cells can also become neurons, astrocytes, or oligodendrocytes when treated with the proper growth factors.
What are Stem Cells, and What Does the “Stem” Part Mean?
Stem cells are immature or “unspecialized” cells. Similar to a blank sheet of paper, they are like blank cells that can become the different types of specialized cells. Stem cells can keep dividing as long as they are alive (we say they are “self-renewing”), and they have two important features: they can create other stem cells and they can become multiple types of more specialized cells (Figure 2). In the brain, we have neural stem cells. That means that these neural stem cells can give rise to neurons, astrocytes, or oligodendrocytes. There are many stem cells in the brain of an embryo because the neural stem cells give rise to all the cell types of the brain; the majority of brain cells are born in the embryo stage. Interestingly, neural stem cells persist in the brain even into adulthood, where they are located in specific parts of the brain (Figure 3).
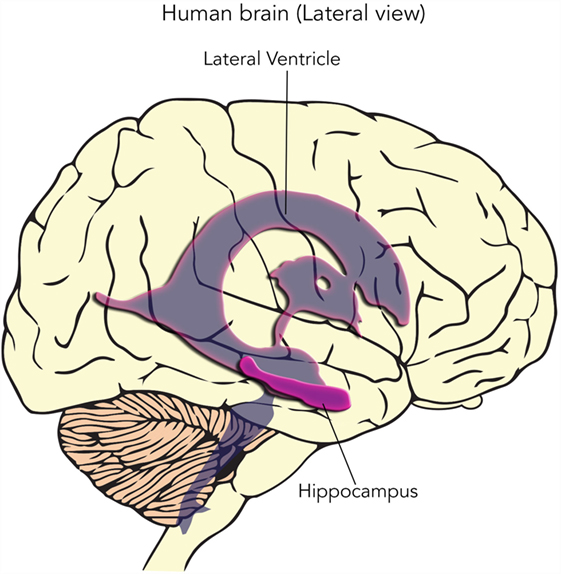
- Figure 3
- In this side view of the human brain, the location of brain areas called the hippocampus and lateral ventricles are shown, deep inside the brain. Scientists have found neural stem cells in these two regions of the brain.
The history of the term “stem” cell is rather complicated. A simplified analogy is to think of the stem of a tree, from which all the different branches arise. In much the same way, a stem cell is a cell from which all different types of specialized cells arise.
When Did Researchers First Discover Stem Cells in the Adult Brain?
The discovery of stem cells in the adult brain took several decades and many different scientists were involved (Figure 4). Here, we provide a summary of several important discoveries. Please note that many other groups and studies also made important contributions and that these can also be found by researching the literature. In the 1960s, two researchers working together, Dr. Joseph Altman and Dr. Gopal D. Das, presented early evidence for the presence of neural stem cells in the brains [1, 2], followed by several other researchers in the 1980s [3, 4]. When two different groups were able to actually isolate stem cells from brains and demonstrate that these cells exhibited the stem cell properties described above, scientists really began to take notice.
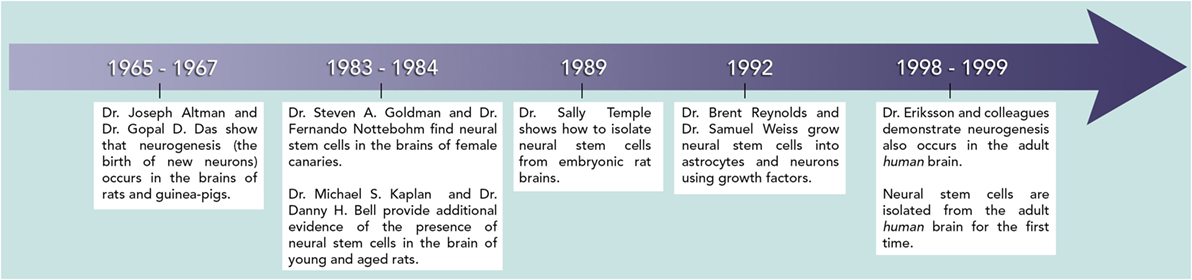
- Figure 4 - This timeline shows when the pioneering discoveries in neural stem cell research occurred.
- These contributions set the basis for today’s advances in the field.
Dr. Sally Temple isolated stem cells from embryonic brains of rats in 1989 [5], and she was able to grow them in cell culture dishes in the lab. Some of the stem cells she found could become neurons and other stem cells could become astrocytes. All of the stem cells could make more neural stem cells. Her work made scientists think that similar stem cells could be found in the embryonic brains of other mammals, including humans. In 1992, Dr. Brent Reynolds and Dr. Samuel Weiss showed that adult mouse brains also contain stem cells [6]. They grew the cells from adult mouse brains in a dish with some important molecules called “growth factors” (substances that tell cells to grow and divide). They showed that in the presence of these growth factors, the isolated cells could both self-renew and become neurons or astrocytes, meaning they were neural stem cells. Building on these results, researchers more recently did experiments to discover exactly when new neurons are born from neural stem cells in the brains of adult rats [7] and humans [8], allowing us to understand even more about this type of stem cells, including important differences between humans and rodents that are beyond the scope of this article.
How Might Understanding More about Neural Stem Cells Help Us to Treat Injury or Diseases of the Brain?
Scientists are actively studying how neural stem cells (either those already existing in the brain or those grown in a laboratory or taken from another brain) can help to treat things such as stroke (when normal blood flow to the brain stops and therefore cells cannot get enough nutrients and oxygen), spinal cord injury, and Parkinson’s disease (a disease in which cells that contribute to control body movements progressively stop working and die). Neural stem cells in the brain are really sensitive to change. For example, after a brain injury, neural stem cells will travel through the brain tissue right to the site of the injury. We know that this actually improves recovery [9], because when scientists prevent the stem cells from moving to the injury site, recovery is much worse [10]. The mechanisms explaining how and why stem cells help recovery from brain injury are an important and active area of research. Neural stem cells are also affected in some brain diseases, such as Parkinson’s and Alzheimer’s. In these diseases, neural stem cells seem to have lower proliferation rates and are less likely to become fully developed and healthy neurons.
Scientists can also inject stem cells from another source into the body (through the blood stream or directly into the brain or spinal cord) and look to see if recovery from a brain injury improves. Several research groups are working on the details of this treatment and carefully studying the outcomes. Eventually, if either potentiation of neural stem cells residing in the brain or the transfer of those grown in a laboratory becomes successful and reproducible, doctors will have better methods for treating several different types of brain injuries or disorders. In the meantime, thanks to the very important discovery of neural stem cells; we will continue to understand the brain, its functions, and design different ways to make it healthier.
Glossary
Cell: ↑ Smallest unit of life – consisting of membrane enclosed sac that contains organelles to ensure survival.
Deoxyribonucleic acid (DNA): ↑ (/de-oxi-ribo-nu-cleic + acid) Instruction code contained in each cell that tells the cell how to build and maintain the organism.
Specialized: ↑ A type of cell that has adapted to fulfill specific functions by changing its shape and structures.
Unspecialized: ↑ A cell in an immature state with the potential of becoming a specialized type of cell. These cells are like a blank sheet of paper.
Embryonic: ↑ A very early stage of development where an organism is just starting to grow the major structures of organs and systems.
Alzheimer’s disease: ↑ (/alt-zhi-merz/ + disease) A disease of the brain that leads to memory loss and mental impairments.
Proliferation rate: ↑ How fast the number of cells is increasing when they multiply.
Potentiation: ↑ To increase the strength, function, or response of a living subject or object.
Reproducible: ↑ Refers to the ability to repeat a task and obtain the same result every time.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
[1] ↑ Altman, J., and Das, G. D. 1965. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124(3):319–35. doi:10.1002/cne.901240303
[2] ↑ Altman, J., and Das, G. D. 1966. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J. Comp. Neurol. 126(3):337–89.
[3] ↑ Goldman, S. A., and Nottebohm, F. 1983. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. U.S.A. 80(8):2390–4. doi:10.1073/pnas.80.8.2390
[4] ↑ Kaplan, M. S., and Bell, D. H. 1984. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J. Neurosci. 4(6):1429–41.
[5] ↑ Temple, S. 1989. Division and differentiation of isolated CNS blast cells in microculture. Nature 340(6233):471–3. doi:10.1038/340471a0
[6] ↑ Reynolds, B. A., and Weiss, S. 1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255(5052):1707–10. doi:10.1126/science.1553558
[7] ↑ Cameron, H. A., Woolley, C. S., McEwen, B. S., and Gould, E. 1993. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56(2):337–44. doi:10.1016/0306-4522(93)90335-D
[8] ↑ Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., et al. 1998. Neurogenesis in the adult human hippocampus. Nat. Med. 4(11):1313–7. doi:10.1038/3305
[9] ↑ Thored, P., Arvidsson, A., Cacci, E., Ahlenius, H., Kallur, T., Darsalia, V., et al. 2006. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24(3):739–47. doi:10.1634/stemcells.2005-0281
[10] ↑ Jin, K., Wang, X., Xie, L., Mao, X. O., and Greenberg, D. A. 2010. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc. Natl. Acad. Sci. U.S.A. 107(17):7993–8. doi:10.1073/pnas.1000154107